199982
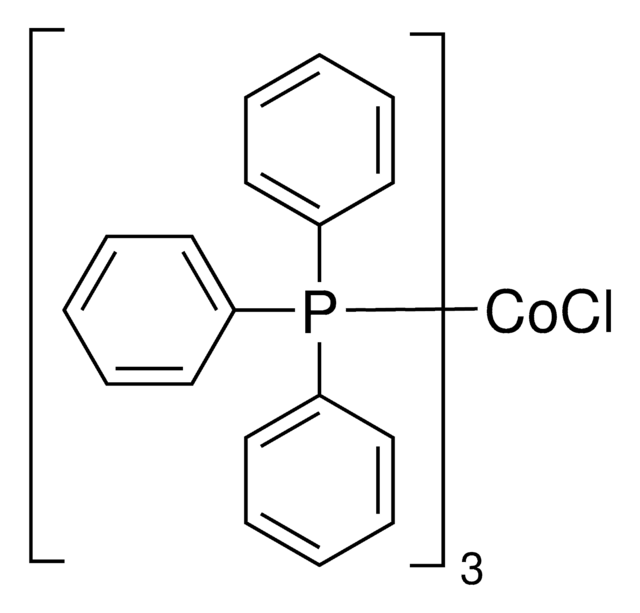
Tris(triphenylphosphine)rhodium(I) chloride
Synonym(s):
NSC 124140, RhCl(PPh3)3, Rhodium(I) tris(triphenylphosphine) chloride, Wilkinson’s catalyst
About This Item
Recommended Products
reaction suitability
core: rhodium
reagent type: catalyst
SMILES string
Cl[Rh].c1ccc(cc1)P(c2ccccc2)c3ccccc3.c4ccc(cc4)P(c5ccccc5)c6ccccc6.c7ccc(cc7)P(c8ccccc8)c9ccccc9
InChI
1S/3C18H15P.ClH.Rh/c3*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;/h3*1-15H;1H;/q;;;;+1/p-1
InChI key
IXAYKDDZKIZSPV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
Catalyst used for many organic reactions including:
- Chemoselective allylic alkylations
- Stoichiometric activation of Si-H bonds and hydrosilylations
- Inter- and intramolecular hydroacylation of alkenes along with a cocatalyst
- Polymerization of diorganostannanes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Trost group's protocol yields α-vinylsilanes from terminal acetylenes using [Cp*Ru(MeCN)3]PF6 catalyst and others for hydrosilylation.
Trost group's protocol yields α-vinylsilanes from terminal acetylenes using [Cp*Ru(MeCN)3]PF6 catalyst and others for hydrosilylation.
Trost group's protocol yields α-vinylsilanes from terminal acetylenes using [Cp*Ru(MeCN)3]PF6 catalyst and others for hydrosilylation.
Trost group's protocol yields α-vinylsilanes from terminal acetylenes using [Cp*Ru(MeCN)3]PF6 catalyst and others for hydrosilylation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




rhodium(I) tetrafluoroborate 98%](/deepweb/assets/sigmaaldrich/product/structures/138/264/047825b4-1f5a-486a-9f51-8d9c18b1382f/640/047825b4-1f5a-486a-9f51-8d9c18b1382f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)