192074
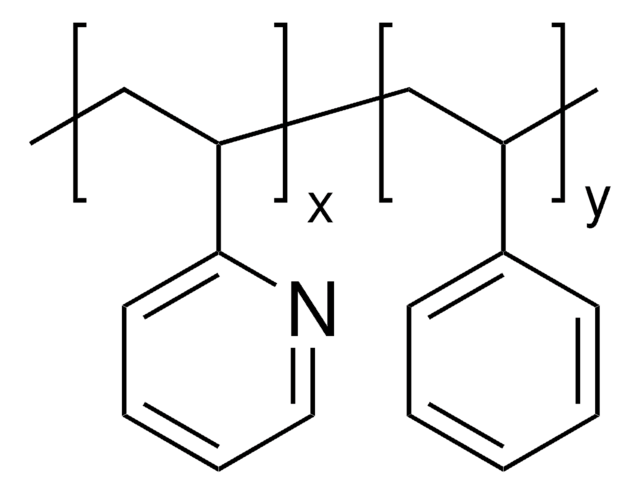
Poly(4-vinylpyridine-co-styrene)
powder
Synonym(s):
4-Vinylpyridine-styrene copolymer, Poly(4-vinylpyridine)-polystyrene copolymer, Styrene-4-vinylpyridine copolymer
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
form
powder
Quality Level
application(s)
advanced drug delivery
coatings
SMILES string
C=CC1=CC=CC=C1.C=CC1=CC=NC=C1
InChI
1S/C8H8.C7H7N/c1-2-8-6-4-3-5-7-8;1-2-7-3-5-8-6-4-7/h2-7H,1H2;2-6H,1H2
InChI key
CIPXQVPZUWMSQE-UHFFFAOYSA-N
Application
Poly(4-vinylpyridine-co-styrene) can be used:
- As a precursor in the preparation of ionic polymer with pendant photochromic moieties by reactingwith1,3,3-trimethyl-6′-bromohexyloxyspiro[2H]-indol-2,3′-[3H]-naphth[2,1-b][1,4]oxazine.
- As permselective membrane in the preparation of composite electrodes for glucose sensing.
- To prepare mixed redox polymer [RuIII(edta)(OH2)(py)]2[RuII(NH3)5(py)], which can switch into six redox states.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yeong-Soon Gal et al.
Journal of nanoscience and nanotechnology, 8(9), 4885-4888 (2008-12-04)
An ionic polymer with pendant photochromic moieties was prepared by the reaction of poly(4-vinylpyridine-co-styrene) and 1,3,3-trimethyl-6'-bromohexyloxyspiro[2H]-indol-2,3'-[3H]-naphth[2,1-b][1,4]oxazine. The photochromic reaction in question is caused by the reversible heterolytic cleavage of the C(spiro)-O bond under UV irradiation, yielding the colored form that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service