All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5ClN2O2
CAS Number:
Molecular Weight:
160.56
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
257 °C (dec.) (lit.)
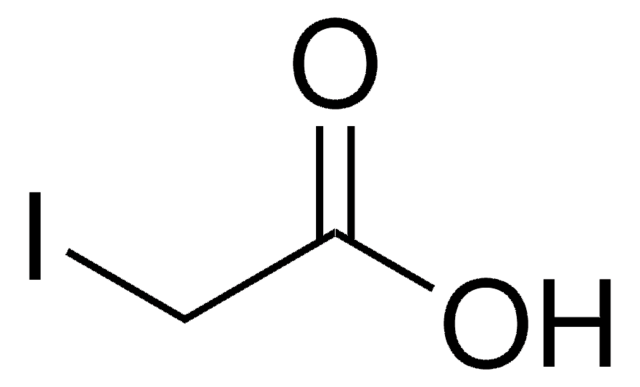
SMILES string
ClCC1=CC(=O)NC(=O)N1
InChI
1S/C5H5ClN2O2/c6-2-3-1-4(9)8-5(10)7-3/h1H,2H2,(H2,7,8,9,10)
InChI key
VCFXBAPEXBTNEA-UHFFFAOYSA-N
General description
6-(Chloromethyl)uracil on chlorination with sulfuryl chloride in acetic acid yields 5-chloro-6-(chloromethyl)uracil.
Application
6-(Chloromethyl)uracil was used in the synthesis of:
- 5-bromo-6-(chloromethyl)uracil
- pteridine compounds, potential anticancer agents
- substituted uracil pyridinium compounds, potential inhibitors of thymidine phosphorylase
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Paul E Murray et al.
Bioorganic & medicinal chemistry, 10(3), 525-530 (2002-01-30)
A series of water soluble N(1)- and C(6)-substituted uracil pyridinium compounds were prepared as potential inhibitors of thymidine phosphorylase (TP). The C(6)-uracil substituted derivatives were the most active. 1-[(5-Chloro-2,4-dihydroxypyrimidin-6-yl)methyl]pyridinium chloride, was identified as the best inhibitor being 5-fold more potent
Shingo Yano et al.
Bioorganic & medicinal chemistry, 12(13), 3431-3441 (2004-06-10)
A series of novel 6-methylene-bridged uracil derivatives have been prepared as inhibitors of human thymidine phosphorylase (TP). To enhance the in vivo antitumor activity of fluorinated pyrimidine 2'-deoxyribonucleosides such as 2'-deoxy-5-(trifluoromethyl)uridine (F(3)dThd), a potent TP inhibitor preventing their degradation to
Prem M S Chauhan et al.
Bioorganic & medicinal chemistry, 13(10), 3513-3518 (2005-04-26)
Several pteridine analogues 4-13, 23-26 have been synthesized and tested in vitro against three cancer cell lines, MCF7 (breast), NCI-H460 (lung) and SF-268 (CNS). All tested pteridines can serve as novel templates for the anticancer chemotherapy and can serve as
W Wang et al.
International journal of radiation biology, 71(4), 387-399 (1997-04-01)
In this work radicals generated by dissociative electron attachment to iodoacetamide (H2NCOCH2.) and 6-chloromethyluracil (U5CH2.) are suggested to react with DNA nucleotides in frozen aqueous solutions via either hydrogen abstraction or addition to the double bonds of the bases. Methyl
J Klosa
Arzneimittel-Forschung, 30(2), 228-231 (1980-01-01)
The synthesis of new uracil derivatives is described. In 4-chloromethyluracil, chlorine can be easily exchanged under mild conditions for amine, aniline, hydrazine, and phenol.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service