164771
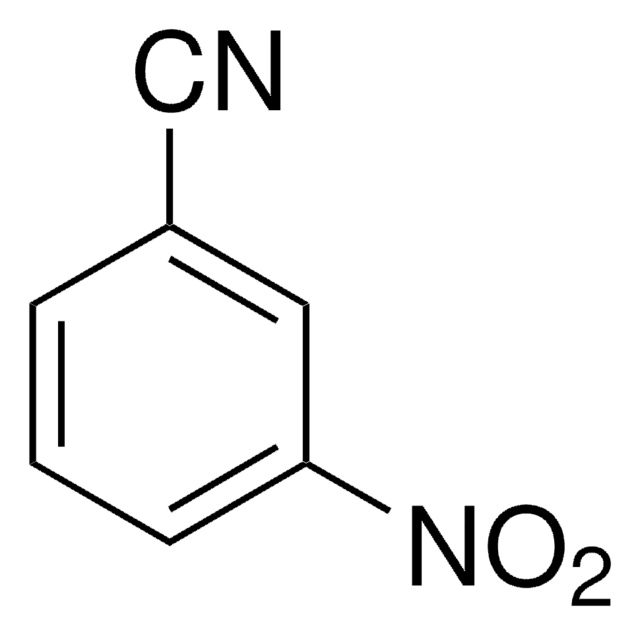
3-Aminobenzonitrile
99%
Synonym(s):
3-Cyanoaniline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H4CN
CAS Number:
Molecular Weight:
118.14
Beilstein:
636498
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
48-53 °C (lit.)
functional group
nitrile
SMILES string
Nc1cccc(c1)C#N
InChI
1S/C7H6N2/c8-5-6-2-1-3-7(9)4-6/h1-4H,9H2
InChI key
NJXPYZHXZZCTNI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Aminobenzonitrile on condensation reaction with 4-isothiocyanato-4-methyl pentane-2-one gives condensed monocyclic pyrimidine derivatives.
Application
3-Aminobenzonitrile was used in the synthesis of series of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles. It was also used in the preparation of highly substituted γ-lactam analogues of a thiazolidinone follicle stimulating hormone receptor agonist.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cheng Hua Jin et al.
European journal of medicinal chemistry, 46(9), 3917-3925 (2011-06-24)
A series of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles 14a-d, 15a-d, 17a, 17b, 18a-d, 19a, and 19b has been synthesized and evaluated for their ALK5 inhibitory activity in an enzyme assay and in a cell-based luciferase reporter assay. The 2-[3-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl]-N-phenylethanethioamide (18a) inhibited ALK5 phosphorylation with
Jeffrey C Pelletier et al.
Bioorganic & medicinal chemistry, 13(21), 5986-5995 (2005-08-16)
An unusual combination of Weinreb amidation and Mitsunobu lactam formation was used to prepare highly substituted gamma-lactam analogues of a thiazolidinone follicle stimulating hormone receptor agonist. The analogue synthesis was stereoselective and the final products were chemically stable. Biological properties
Sham M Sondhi et al.
Bioorganic & medicinal chemistry, 13(22), 6158-6166 (2005-08-24)
3-Aminobenzonitrile and 2-amino-4-phenyl thiazole on condensation with 4-isothiocyanato-4-methyl pentane-2-one gave condensed monocyclic pyrimidine derivatives 1 and 2, 3, respectively. Condensation of 3-aminopropyl imidazole with 3-isothiocyantobutanal gave condensed monocyclic pyrimidine derivative 4. Bicyclic pyrimidine derivatives 5a and 5b have been synthesized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service