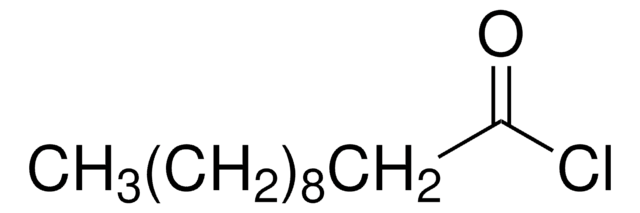156833
Nonanoyl chloride
96%
Synonym(s):
Pelargonyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)7COCl
CAS Number:
Molecular Weight:
176.68
Beilstein:
1362621
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.438 (lit.)
bp
108-110 °C/22 mmHg (lit.)
density
0.98 g/mL at 25 °C (lit.)
functional group
acyl chloride
SMILES string
CCCCCCCCC(Cl)=O
InChI
1S/C9H17ClO/c1-2-3-4-5-6-7-8-9(10)11/h2-8H2,1H3
InChI key
NTQYXUJLILNTFH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Nonanoyl chloride was used for the acylation of amine groups of chitosan to simultaneously improve its material properties and adsorption capacity. It was also used in the synthesis of:
- N-nonanoyl piperonylamide
- caged vanilloid, N-(4-hydroxy-3-methoxybenzyl)-N-(2-nitrobenzyl)-nonanoylamide or N-(2-Nitrobenzyl)-N-vanillyl-nonanoylamide
- n-benzylnonanamide
Other Notes
Remainder 2-Methyloctanoyl chloride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
210.2 °F
Flash Point(C)
99 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karen C Thomas et al.
The Journal of pharmacology and experimental therapeutics, 321(3), 830-838 (2007-03-03)
Transient receptor potential vanilloid 1 (TRPV1) is a calcium-selective ion channel expressed in human lung cells. We show that activation of the intracellular subpopulation of TRPV1 causes endoplasmic reticulum (ER) stress and cell death in human bronchial epithelial and alveolar
Effects of Acylation and Crosslinking on the Material Properties and Cadmium Ion Adsorption Capacity of Porous Chitosan Beads.
Hsien, Tzu-Yang, and Gregory L. Rorrer
Separation Science and Technology, 30(12), 2455-2475 (1995)
Hypotensive and antinociceptive effects of ether-linked and relatively non-pungent analogues of< i> N</i>-nonanoyl vanillylamide.
Chen IJ , et al.
European Journal of Medicinal Chemistry, 27(3), 187-192 (1992)
Yannick Hövelmann et al.
Journal of agricultural and food chemistry, 67(13), 3670-3678 (2019-03-13)
Imidazole alkaloids represent a rather small group of alkaloids and are assumed not to be of significance to the human food chain so far. In this study, novel imidazole alkaloids occurring in tomato products were synthesized and structurally characterized by
Jun Zhao et al.
Biochemistry, 45(15), 4915-4926 (2006-04-12)
Nociceptive neurons in the peripheral nervous system detect noxious stimuli and report the information to the central nervous system. Most nociceptive neurons express the vanilloid receptor, TRPV1, a nonselective cation channel gated by vanilloid ligands such as capsaicin, the pungent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service