125504
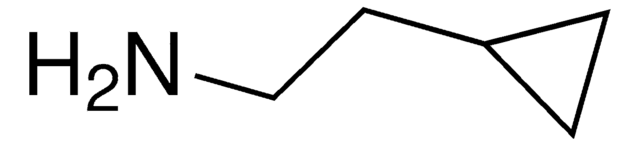
Cyclopropylamine
98%
Synonym(s):
Aminocyclopropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C3H5NH2
CAS Number:
Molecular Weight:
57.09
Beilstein:
741858
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
4.67 psi ( 20 °C)
Quality Level
Assay
98%
form
liquid
autoignition temp.
527 °F
refractive index
n20/D 1.420 (lit.)
bp
49-50 °C (lit.)
density
0.824 g/mL at 25 °C (lit.)
SMILES string
NC1CC1
InChI
1S/C3H7N/c4-3-1-2-3/h3H,1-2,4H2
InChI key
HTJDQJBWANPRPF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclopropylamine(CPA) has been used in the synthesis of N-[4-(4-fluoro)phenyl-2-aminothiazol-5-yl]pyrimidin-2-yl-alkylamine derivatives. It has been used in the synthesis of Pt(CPA)2(bismethylthiomethylenepropanedioate) and Pt(CPA)2(bisethylthiomethylenepropanedioate) complexes.
Biochem/physiol Actions
Cyclopropylamine inactivates cytochrome P450 enzymes by a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme. It is a mechanism-based inhibitor of quinoprotein methylamine dehydrogenase from Paracoccus denitrificans.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
33.8 °F - closed cup
Flash Point(C)
1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dapeng Sun et al.
FEBS letters, 517(1-3), 172-174 (2002-06-14)
Cyclopropylamine is a mechanism-based inhibitor of the quinoprotein methylamine dehydrogenase (MADH) from Paracoccus denitrificans. The resulting inactivation is accompanied by the formation of a covalent cross-link between the alpha and beta subunits of MADH. The results of site-directed mutagenesis studies
Synthesis and Antifungal Activity of 5-[2-(Alkylamino) pyrimidin-4-yl]-4-phenylthiazol-2-cycloalkylamine Derivatives on Phytophthora capsici.
Nam Sw, et al.
J. Korean Chem. Soc., 54(3), 395-402 (2011)
David J Aitken et al.
Organic & biomolecular chemistry, 9(21), 7517-7524 (2011-09-23)
The Kulinkovich-de Meijere reaction between an unsaturated Grignard reagent and a chiral amide takes place with a high trans stereoselectivity and provides a convenient access to non-racemic trans cyclopropylamines. These compounds are transformed in four steps into the corresponding N-protected
Mark E Scott et al.
The Journal of organic chemistry, 73(21), 8154-8162 (2008-10-10)
This manuscript describes a highly selective iodide-mediated, tandem Mannich/cyclization to afford trans-2,3-disubstituted pyrrolidines from methylenecyclopropyl amides in good to excellent yields and selectivities. The reaction scope has been drastically expanded to include a wide array of aromatic, heteroaromatic and alpha,beta-unsaturated
Fluorinated phenylcyclopropylamines as inhibitors of monoamine oxidases.
Thomas C Rosen et al.
Chembiochem : a European journal of chemical biology, 5(8), 1033-1043 (2004-08-10)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service