H0161
Hyaluronic Acid Binding Protein bovine
lyophilized powder
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
bovine
Quality Level
form
lyophilized powder
technique(s)
immunofluorescence: suitable
UniProt accession no.
storage temp.
−20°C
Gene Information
cow ... VCAN(282662)
General description
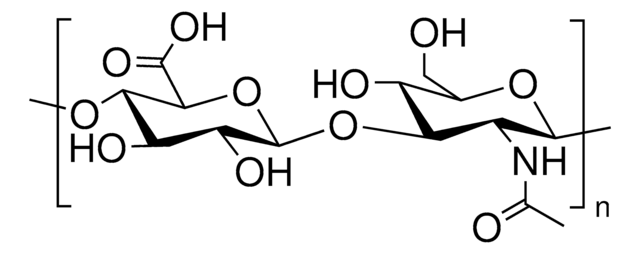
Hyaluronic acid (HA) is a linear polysaccharide consisting of a repeating disaccharide of N-acetyl D-glucosamine (GlcNAc) and D-glucuronic acid (GlcA) with 1→4 interglycosidic linkages and these disaccharide repeating units are in turn linked by β (1→3) linkages. The number of repeating disaccharides in a HA molecule ranges from 2,000 to 25,000, which relates to a molecular mass of 106-107. The unique biological roles of HA is mainly due to the specific binding and interaction with HA-binding proteins also known as hyaladherins. Most hyaladherins is composed of a specific binding domain, also called a link module, which is composed of two α-helices and two antiparallel β-sheets.
Karl Meyer and John Palmer first isolated hyaluronic acid (HA) from the vitreous of bovine eyes. They coined the name “hyaluronic acid” as a conjugation of two words, hyaloid (vitreous) and uronic acid. HA has an extended random coil structure in physiological solution. Due to its random-coil structure and high molecular weight (HMW), HA forms very viscose and elastic solution with a huge hydrodynamic volume.
Application
Hyaluronic Acid Binding Protein bovine may be used for incubation of specimens for immunofluorescence analysis of human megakaryocytes.
Biochem/physiol Actions
Hyaluronic Acid Binding Protein (HABP) levels increases during oocyte maturation and it promotes cumulus expansion during in vitro maturation.
In addition to being an important structural component of tissues in all vertebrates, HA is also associated with several biological functions including intracellular signaling.
May be useful in hyaluronic acid binding or detection.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hyaluronan based hydrogels provide an improved model to study megakaryocyte-matrix interactions
Currao M, et al.
Experimental Cell Research, 346(1), 1-8 (2016)
A G Haus
Radiologic clinics of North America, 25(5), 913-928 (1987-09-01)
Today there are many dedicated mammographic x-ray units available that are capable of providing high-quality screen-film mammograms. Likewise, screen-film combinations designed for mammography are capable of providing images with appropriate contrast, resolution, and noise levels. Proper film processing is most
Identification of hyaluronic acid-binding proteins and their expressions in porcine cumulus-oocyte complexes during in vitro maturation
Yokoo M, et al.
Biology of Reproduction, 67(4), 1165-1171 (2002)
G Perides et al.
The Journal of biological chemistry, 264(10), 5981-5987 (1989-04-05)
A glial hyaluronate-binding protein (GHAP) with an isoelectric point of 4.3-4.4 was isolated from human brain white matter. The 60-kDa glycoprotein appeared to be quite resistant to proteolysis, and comparison with GHAP from a viable glioma removed at surgery showed
Tal M Dankovich et al.
Nature communications, 12(1), 7129-7129 (2021-12-10)
The brain extracellular matrix (ECM) consists of extremely long-lived proteins that assemble around neurons and synapses, to stabilize them. The ECM is thought to change only rarely, in relation to neuronal plasticity, through ECM proteolysis and renewed protein synthesis. We
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service