78194
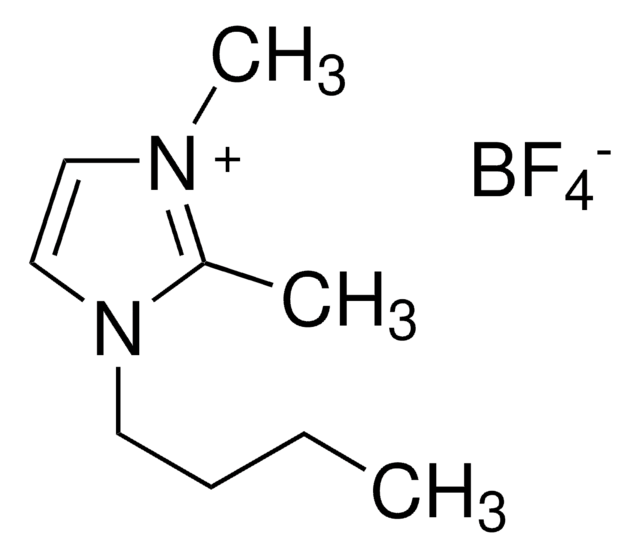
1-Butyl-2,3-dimethylimidazolium chloride
≥97.0% (HPLC/AT)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H17ClN2
CAS Number:
Molecular Weight:
188.70
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (HPLC/AT)
form
crystals
impurities
≤1.0% water
SMILES string
[Cl-].CCCCn1cc[n+](C)c1C
InChI
1S/C9H17N2.ClH/c1-4-5-6-11-8-7-10(3)9(11)2;/h7-8H,4-6H2,1-3H3;1H/q+1;/p-1
InChI key
HHHYPTORQNESCU-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-Butyl-2,3-dimethylimidazolium chloride can be used:
- As a solvent in the chemical modification of polysaccharide cellulose.
- As a model ionic liquid in the conversion of a monosaccharide like fructose into 5-hydroxymethylfurfural using H2SO4.
- To prepare 1-butyl-2,3-dimethylimidazolium dicarba-7,8-nidoundecaborate by reacting with caesium dicarba-7,8-nido-undecaborate.
- To prepare mesoporous ZnAl2O4 nanomaterials, which are used as catalysts or catalyst supports.
Physical form
ionic liquid
Other Notes
Ionic liquid suitable for use in strongly basic conditions, e.g. for oxidations and metathesis reactions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Interactions of ionic liquids with polysaccharides-2: Cellulose
Heinze T, et al.
Macromolecular Symposia, 262(1), 8-22 (2008)
Yanqi Wang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(7), 1624-1632 (2020-01-24)
Although supertetrahedral Tn sulfide clusters (n=2-6) have been extensively explored, the synthesis of Tn selenide clusters with n>4 has not been achieved thus far. Reported here are ionic-liquid (IL)-assisted precursor route syntheses, characterizations, and the photocatalytic properties of six new
Bertram Peters et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(70), 16683-16689 (2020-09-03)
Multinary chalcogenido (semi)metalate salts exhibit finely tunable optical properties based on the combination of metal and chalcogenide ions in their polyanionic substructure. Here, we present the structural expansion of chalcogenido germanate(IV) or stannate(IV) architectures with SbIII , which clearly affects
Preparation of mesoporous ZnAl2O4 nanoflakes by ion exchange from a Na-dawsonite parent material in the presence of an ionic liquid
Kim T, et al.
Royal Society of Chemistry Advances, 9(21), 11894-11900 (2019)
Crystal structure of 1-butyl-2, 3-dimethylimidazolium dicarba-7, 8-nido-undecaborate
Klemes MJ, et al.
Acta Crystallographica Section E: Crystallographic Communications, 71(3), o183-o183 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service