496383
1,10-Phenanthroline-5,6-dione
97%
Synonym(s):
Stahl phd oxidant, phd
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H6N2O2
CAS Number:
Molecular Weight:
210.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
260 °C (dec.) (lit.)
SMILES string
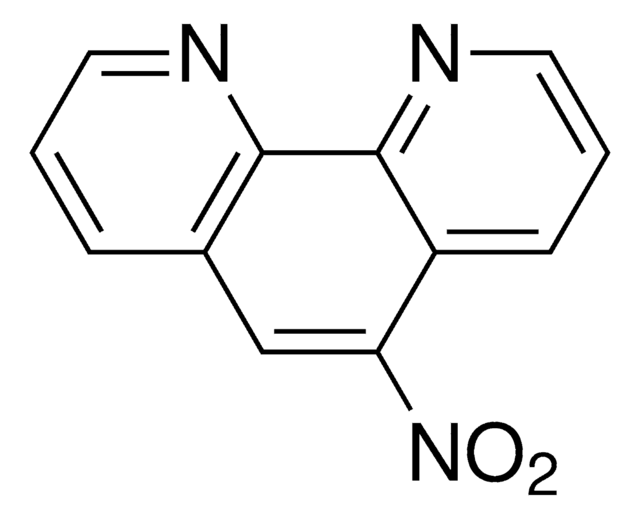
O=C1C(=O)c2cccnc2-c3ncccc13
InChI
1S/C12H6N2O2/c15-11-7-3-1-5-13-9(7)10-8(12(11)16)4-2-6-14-10/h1-6H
InChI key
KCALAFIVPCAXJI-UHFFFAOYSA-N
General description
1,10-Phenanthroline-5,6-dione (phendio) forms Cu(II) and Ag(I) phendio complexes, which show potent anti-fungal and anti-cancer activity. The modification of glassy carbon (GC) electrodes with phendio complexes of transition metals leads to the catalytic oxidation of NADH at low overpotential.
Application
1,10-Phenanthroline-5,6-dione may be used in the preparation of homo- and heterometallic complexes with early transition metal ions.
A Bifunctional quinone oxidant which, when used in conjunction with Zn2+ catalysts, is used to affect the aerobic oxidation of secondary amines to a variety of value added motifs, including indoles.
Bioinspired Aerobic Oxidation of Secondary Amines and Nitrogen Heterocycles with a Bifunctional Quinone Catalyst
Bioinspired Aerobic Oxidation of Secondary Amines and Nitrogen Heterocycles with a Bifunctional Quinone Catalyst
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yu-Min Song et al.
Journal of inorganic biochemistry, 103(3), 396-400 (2009-01-13)
Three new solid complexes have been synthesized by the reaction of rare earth(III) nitrate with the first ligand curcumin (HL) and the second ligand 1,10-phenanthroline-5,6-dione (L') in alcohol solution (pH=6.5-7.0). The composition of the complexes has been characterized by elemental
Electrocatalytic oxidation of NADH at glassy carbon electrodes modified with transition metal complexes containing 1, 10-phenanthroline-5, 6-dione ligands.
Wu Q, et al.
Analytical Chemistry, 68(20), 3688-3696 (1996)
1, 10-Phenanthroline-5, 6-dione as a building block for the synthesis of homo-and heterometallic complexes.
Calderazzo F, et al.
Inorgorganica Chimica Acta, 330(1), 136-142 (2002)
Aleksandra Pinczewska et al.
Journal of the American Chemical Society, 134(43), 18022-18033 (2012-10-11)
We report the combinatorial preparation and high-throughput screening of a library of modified electrodes designed to catalyze the oxidation of NADH. Sixty glassy carbon electrodes were covalently modified with ruthenium(II) or zinc(II) complexes bearing the redox active 1,10-phenanthroline-5,6-dione (phendione) ligand
Xuyan Mao et al.
The Analyst, 136(2), 293-298 (2010-10-20)
To improve the electrocatalytic activities of carbon nanotubes (CNT) towards the oxidation of nicotinamide adenine dinucleotide (NADH), we derive them with a redox mediator, 1,10-phenanthroline-5,6-dione (PD), by the noncovalent functionalization method. The redox carbon nanotubes (PD/CNT/GC) show excellent electrocatalytic activities
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service