252581
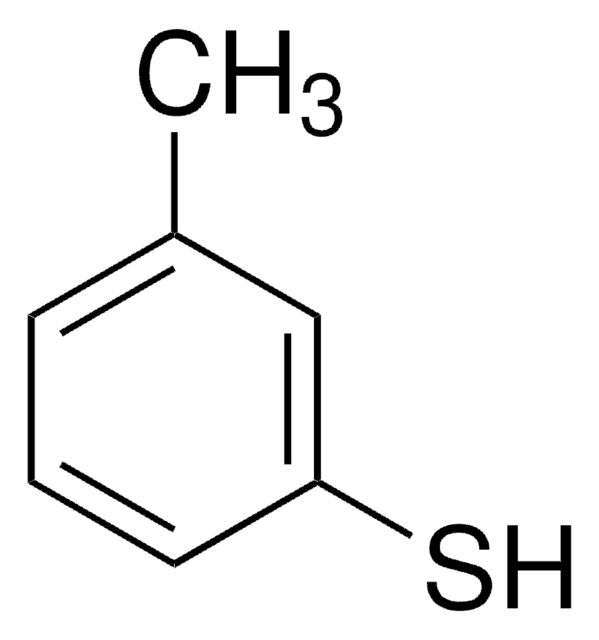
2-Phenylethanethiol
98%
Synonym(s):
2-Phenylethyl mercaptan, Phenethyl mercaptan
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2CH2SH
CAS Number:
Molecular Weight:
138.23
Beilstein:
1446618
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.560 (lit.)
bp
217-218 °C (lit.)
density
1.032 g/mL at 25 °C (lit.)
functional group
phenyl
thiol
SMILES string
SCCc1ccccc1
InChI
1S/C8H10S/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2
InChI key
ZMRFRBHYXOQLDK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Phenylethanethiol serves as a building block in organic synthesis to incorporate a thiol functional group into molecules. Structures of 2-phenylethanethiol and its 1:1 water clusters have been studied using resonant two-photon ionization spectroscopy.
Application
2-Phenylethanethiol has been used:
- in preparation of biicosahedral Au (25) clusters protected with various types of thiol ligands
- as derivatization reagent for phosphorylated sequences of peptides, for enhancing ionization efficiency in matrix-assisted laser desorption/ionization mass spectrometry
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
SunYoung Park et al.
Langmuir : the ACS journal of surfaces and colloids, 28(17), 7049-7054 (2012-04-12)
The synthesis and electrochemical and spectroscopic characterization of biicosahedral Au(25) clusters with a composition of [Au(25)(PPh(3))(10)(thiolate)(5)Cl(2)](2+) are described. The biicosahedral Au(25) clusters protected with various types of thiol ligands including alkanethiols, 2-phenylethanethiol, 11-mercaptoundecanoic acid, and 11-mercapto-1-undecanol were synthesized in high
Differential reactivity of the inner and outer positions of Au 25 (SCH 2 CH 2 Ph) 18 dimeric staples under place exchange conditions
P Pengo, et al.
Chemical Communications (Cambridge, England), 51, 3204-3207 (2015)
Clementine Klemm et al.
Rapid communications in mass spectrometry : RCM, 18(22), 2697-2705 (2004-10-16)
The identification of phosphorylation sites is essential for a full understanding of the cellular functions of proteins. However, mass spectrometric analysis is often hampered by the low abundance of phosphoproteins, the difficulty of obtaining full sequence coverage by specific proteolysis
Danielle E Martin et al.
The Journal of chemical physics, 128(16), 164301-164301 (2008-05-02)
The structures of 2-phenylethanethiol (PET, PhCH(2)CH(2)SH) and its 1:1 water clusters have been studied using resonant two-photon ionization spectroscopy including band contour analysis and UV-UV holeburning, combined with extensive ab initio calculations on ground and excited states. The most populated
Sanghwa Lee et al.
Journal of the American Chemical Society, 142(32), 13974-13981 (2020-07-17)
Atomically precise coinage metal (Au, Ag, and Cu) nanoclusters (NCs) have been the subject of immense interest for their intriguing structural, photophysical, and catalytic properties. However, the synthesis of Cu NCs is highly challenging because of low reduction potential and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service