163872
Ethyl malonyl chloride
technical grade
Synonym(s):
Ethyl (chloroformyl)acetate, Ethyl 3-chloro-3-oxopropionate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
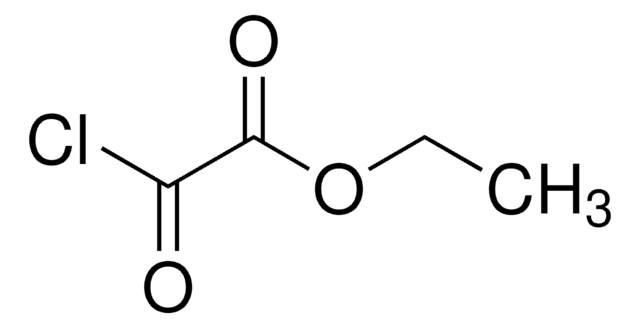
Linear Formula:
CH3CH2OCOCH2COCl
CAS Number:
Molecular Weight:
150.56
Beilstein:
636215
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
refractive index
n20/D 1.429 (lit.)
bp
79-80 °C/25 mmHg (lit.)
density
1.176 g/mL at 25 °C (lit.)
functional group
acyl chloride
ester
storage temp.
2-8°C
SMILES string
CCOC(=O)CC(Cl)=O
InChI
1S/C5H7ClO3/c1-2-9-5(8)3-4(6)7/h2-3H2,1H3
InChI key
KWFADUNOPOSMIJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl malonyl chloride is a versatile acylating agent for propargyl alcohols, hydrazines and amines.
Application
Ethyl malonyl chloride was used in the synthesis of:
- liquid-crystalline methanofullerodendrimers
- 3,5-disubstituted 1,2,4-oxadiazole derivatives, which are potential peptidomimetic building blocks
- 3-pyrrolin-2-ones via amidation with propargylamines and subsequent base catalyzed 5-exo-dig cyclization
Versatile acylating agent for propargyl alcohols, hydrazines, and amines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
147.2 °F - closed cup
Flash Point(C)
64 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 3, 5-disubstituted 1, 2, 4-oxadiazoles as peptidomimetic building blocks.
Jakopin Z, et al.
Tetrahedron Letters, 48(8), 1465-1468 (2007)
Synthesis, 2019-2019 (2006)
J T Gau et al.
Cancer research, 57(17), 3830-3834 (1997-09-01)
Prostate-specific antigen (PSA) has been demonstrated to release the active form of insulin-like growth factor I in vitro (P. Cohen et al., J. Clin. Endocrinol. & Metab., 75: 1046-1053, 1992; P. Cohen et al., J. Clin. Endocrinol. & Metab., 79:
N A Meanwell et al.
Journal of medicinal chemistry, 36(24), 3871-3883 (1993-11-26)
The 4,5-diphenyloxazole derivatives 2-4 were previously identified as nonprostanoid prostacyclin (PGI2) mimetics. A series of derivatives of 2-4 bearing substitutents at the carbon atom alpha to the oxazole ring were synthesized and evaluated as inhibitors of ADP-induced aggregation of human
Synlett, 65-65 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service