148113
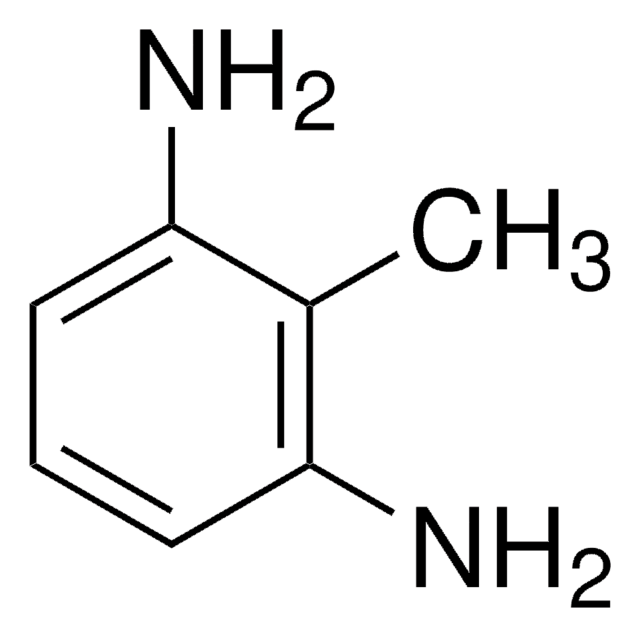
2,6-Diaminotoluene
97%
Synonym(s):
2,6-Toluenediamine, 2,6-Tolylenediamine, 2-Methyl-m-phenylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H3(NH2)2
CAS Number:
Molecular Weight:
122.17
Beilstein:
2079476
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
mp
104-106 °C (lit.)
SMILES string
Cc1c(N)cccc1N
InChI
1S/C7H10N2/c1-5-6(8)3-2-4-7(5)9/h2-4H,8-9H2,1H3
InChI key
RLYCRLGLCUXUPO-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Muta. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Tinnerberg et al.
American Industrial Hygiene Association journal, 58(3), 229-235 (1997-03-01)
Comparative air measurements of toluene diisocyanate (TDI) were performed in a 5.6 m3 standard atmosphere and at a TDI flexible foam plant. Air samples were collected in midget impinger flasks containing 9-(N-methyl-amino-methyl)-anthracene (MAMA) in toluene and on 13-mm glass-fiber filters
P M Wilson et al.
Archives of toxicology, 70(10), 591-598 (1996-01-01)
Using gas chromatography/mass spectrometry for detection of hemoglobin adducts, and 32P-postlabelling for DNA adducts, we examined macromolecular binding in Fischer-344 rats administered 2,4-or 2,6-toluene diamine (TDA). The dose-response and correlative relationship between the two macromolecules were investigated over a range
Hironao Takasawa et al.
Mutation research, 751(1), 12-18 (2012-11-06)
Detecting genotoxicity in the liver is considered an effective approach for predicting hepatocarcinogenicity, as many genotoxic chemicals in vivo may act as hepatocarcinogens in rodents. Here, a genotoxic rodent hepatocarcinogen, 1,2-dimethylhydrazine dihydrochloride (1,2-DMH), and a genotoxic (Ames positive) noncarcinogen, 2,6-diaminotolunene
M L Cunningham et al.
Environmental health perspectives, 104 Suppl 3, 683-686 (1996-05-01)
The aromatic amines 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) are structural isomers that have been extensively studied for their mutagenic and carcinogenic characteristics. Both compounds are rapidly absorbed after oral administration and are equally mutagenic in the Ames test; however, 2,4-DAT
P Lind et al.
The Analyst, 122(1), 51-56 (1997-01-01)
Blood and urine samples were collected from six workers and two volunteers exposed to thermal degradation products from toluene diisocyanate (TDI)-based polyurethane (PUR) before and during the summer vacation. Air samples were collected on filters impregnated with 9-(N-methylaminomethyl)anthracene. The concentrations
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service