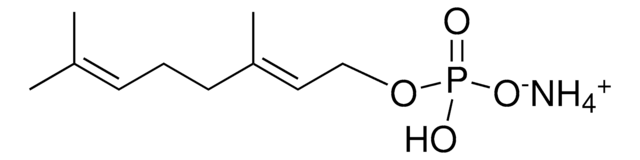
12436
Neryl pyrophosphate lithium salt
≥95.0% (TLC)
Synonym(s):
(Z)-3,7-Dimethyl-2,6-octadien-1-yl pyrophosphate lithium salt, Neryl diphosphate lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H20O7P2 · xLi+
CAS Number:
Molecular Weight:
314.21 (free acid basis)
UNSPSC Code:
12352107
NACRES:
NA.25
Recommended Products
Assay
≥95.0% (TLC)
storage temp.
−20°C
Application
Neryl pyrophosphate, the cis isomer of geranyl pyrophosphate, may be used to characterize and study the kinetics of enzymes such as 1,8-cineole synthase, farnesyl pyrophosphate synthase, pinene cyclase and geranyl pyrophosphate:sabinene hydrate cyclase.
Biochem/physiol Actions
Metabolite, substrate for monoterpene synthase.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T W Hallahan et al.
Archives of biochemistry and biophysics, 264(2), 618-631 (1988-08-01)
A soluble enzyme preparation from the leaves of sweet marjoram (Majorana hortensis Moench) catalyzes the divalent cation-dependent cyclization of [1-3H]geranyl pyrophosphate to the bicyclic monoterpene alcohols (+)-[6-3H]cis- and (+)-[6-3H]-transsabinene hydrate, providing labeling patterns consistent with current mechanistic considerations. No free
R Croteau et al.
The Journal of biological chemistry, 264(26), 15309-15315 (1989-09-15)
(+)-Pinene cyclase from sage (Salvia officinalis) catalyzes the isomerization and cyclization of geranyl pyrophosphate to (+)-alpha-pinene and (+)-camphene, and to lesser amounts of (+)-limonene, myrcene, and terpinolene, whereas (-)-pinene cyclase from this tissue catalyzes the conversion of the acyclic precursor
R Croteau et al.
Archives of biochemistry and biophysics, 309(1), 184-192 (1994-02-15)
Geranyl pyrophosphate: 1,8-cineole cyclase (cineole synthase) catalyzes the conversion of geranyl pyrophosphate to the symmetrical monoterpene ether 1,8-cineole (1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane) by a process thought to involve the initial isomerization of the substrate to the tertiary allylic isomer, linalyl pyrophosphate, and cyclization
D R Light et al.
The Journal of biological chemistry, 264(31), 18598-18607 (1989-11-05)
A prenyltransferase purified from the commercial rubber tree, Hevea brasiliensis, that elongates existing cis-polyisoprene rubber molecules also catalyzes the formation of all trans-farnesyl pyrophosphate (t,t-FPP) from dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP). In assays of the latter activity trans-geranyl
Old substrates for new enzymes of terpenoid biosynthesis.
Jörg Bohlmann et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(26), 10402-10403 (2009-06-26)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service