Y0000612
Dopexamine dihydrochloride
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
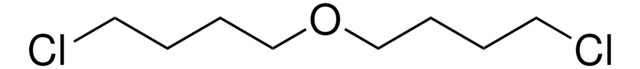
C22H32N2O2 · 2HCl
CAS Number:
Molecular Weight:
429.42
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
dopexamine
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C22H32N2O2.2ClH/c25-21-11-10-20(18-22(21)26)13-17-24-15-7-2-1-6-14-23-16-12-19-8-4-3-5-9-19;;/h3-5,8-11,18,23-26H,1-2,6-7,12-17H2;2*1H
InChI key
VPDULUNRSQWWJB-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Dopexamine dihydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reiner Oberbeck
Current medicinal chemistry, 13(17), 1979-1989 (2006-07-18)
The existence of an immune-endocrine interaction has been reported and the modulatory effects of the natural occurring catecholamines epinephrine, norepinephrine and dopamine as well as of pharmaceutically generated catecholamines like dopexamine on a wide variety of immune functions were demonstrated.
Philippe Seguin et al.
Critical care (London, England), 10(1), R32-R32 (2006-03-02)
Microcirculatory blood flow, and notably gut perfusion, is important in the development of multiple organ failure in septic shock. We compared the effects of dopexamine and norepinephrine (noradrenaline) with those of epinephrine (adrenaline) on gastric mucosal blood flow (GMBF) in
Shaman Jhanji et al.
Critical care (London, England), 14(4), R151-R151 (2010-08-12)
Post-operative outcomes may be improved by the use of flow related end-points for intra-venous fluid and/or low dose inotropic therapy. The mechanisms underlying this benefit remain uncertain. The objective of this study was to assess the effects of stroke volume
N Mayeur et al.
Annales francaises d'anesthesie et de reanimation, 29(11), 759-764 (2010-10-12)
To evaluate the 6 hours haemodynamic effects of dopexamine (DPX) infusion in septic shock patients with persistent hyperlactatemia treated with high dose of norepinephrine (NE). Preliminary, prospective, uncontrolled study. Twenty-one septic shock with NE>0.5 μg/kg/min, venous mixed oxygen saturation (ScvO(2)/SvO(2))>70%
N E El Mokhtari et al.
European journal of medical research, 13(10), 459-463 (2008-11-15)
To examine the effects of a therapy with dopexamine/dopamine in comparison with a regimen of dobutamine/dopamine on the outcome of patients with profound cardiogenic shock. Twenty patients presenting with an acute cardiogenic shock assisted with mechanical ventilation, being refractory to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service