D57558
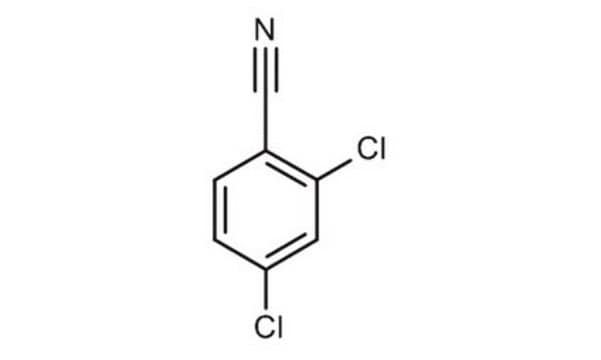
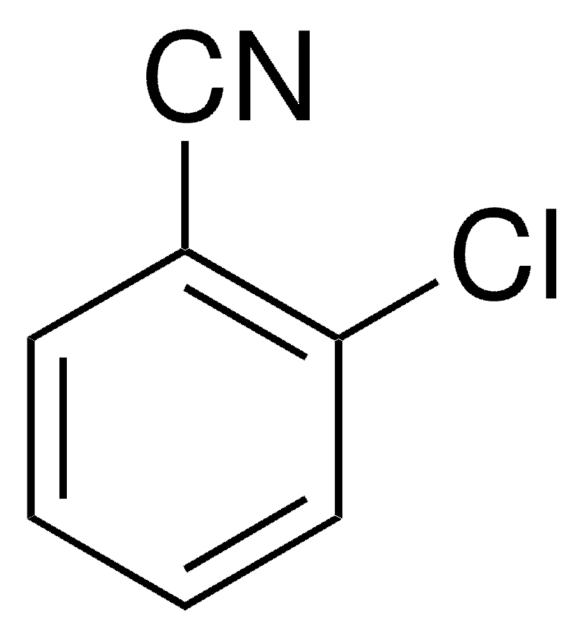
2,6-Dichlorobenzonitrile
97%
Synonym(s):
Dichlobenil
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Cl2C6H3CN
CAS Number:
Molecular Weight:
172.01
Beilstein:
1909167
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
143-146 °C (lit.)
SMILES string
Clc1cccc(Cl)c1C#N
InChI
1S/C7H3Cl2N/c8-6-2-1-3-7(9)5(6)4-10/h1-3H
InChI key
YOYAIZYFCNQIRF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Dichlorobenzonitrile can be used as a starting material to synthesize:
- 2,6-Dichlorobenzaldehyde using lithium N, N′-dimethylethylenediaminoaluminum hydride as a reducing agent.
- 5-(2,6-Dichlorophenyl)-2H-tetrazole via gold-catalyzed nucleophilic (3 + 2) cycloaddition reaction with sodium azide.
- 2,6-Dichlorobenzamide via hydrolysis using potassium tert-butoxide as a catalyst.
- Chloro-aminoindazole by reacting with hydrazine monohydrate.
- 2,6-Dichlorobenzenecarboselenoamide by treating with Woollins′ reagent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of primary arylselenoamides by reaction of aryl nitriles with Woollins' reagent
Hua Guoxiong, et al.
Organic Letters, 8(23), 5251-5254 (2006)
The discovery and development of a safe, practical synthesis of ABT-869
Kruger AW, et al.
Organic Process Research & Development, 13(6), 1419-1425 (2009)
Synthetic application of gold nanoparticles and auric chloride for the synthesis of 5-substituted 1 H-tetrazoles
Kumar S, et al.
Royal Society of Chemistry Advances, 5(28), 21651-21658 (2015)
Transition-metal-free hydration of nitriles using potassium tert-butoxide under anhydrous conditions
Midya GC, et al.
The Journal of Organic Chemistry, 80(8), 4148-4151 (2015)
Selective conversion of aromatic nitriles to aldehydes by lithium N, N'-dimethylethylenediaminoaluminum hydride
Cha Jin-Soon, et al.
Bulletin of the Korean Chemical Society,, 23(12), 1697-1698 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service