706531
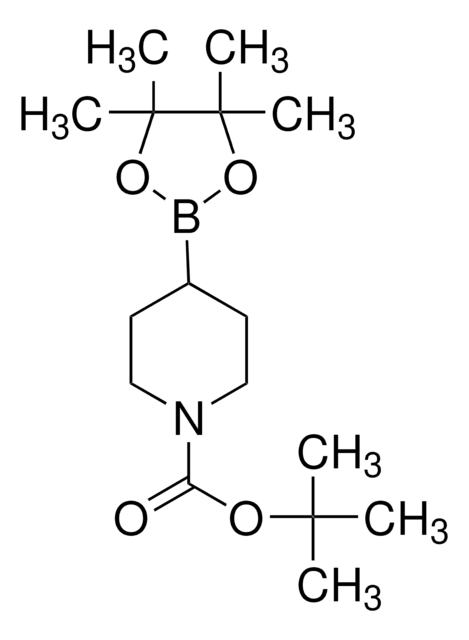
N-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
95%
Synonym(s):
(1-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,5,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl)-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
About This Item
Recommended Products
Quality Level
Assay
95%
form
powder
mp
100-114 °C
SMILES string
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
InChI key
VVDCRJGWILREQH-UHFFFAOYSA-N
Related Categories
General description
Application
- Suzuki-Miyaura cross-coupling using palladium phosphine catalyst
- Palladium-catalyzed ligand-controlled regioselective Suzuki coupling
- Palladium-catalyzed Suzuki-Miyaura coupling
- Suzuki coupling followed by iodolactonization reaction
- Wrenchnolol derivative optimized for gene activation in cells
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Orally active anaplastic lymphoma kinase inhibitors
- Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes
- 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands
- Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder
- Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

