T1143
Trypsinogen from bovine pancreas
essentially salt-free, lyophilized powder, ≥10,000 BAEE units/mg protein (E1%/280, after activation to trypsin)
About This Item
Recommended Products
biological source
bovine pancreas
Assay
85-100% (UV)
form
essentially salt-free, lyophilized powder
specific activity
≥10,000 BAEE units/mg protein (E1%/280, after activation to trypsin)
mol wt
23,981 Da by calculation
technique(s)
mass spectrometry (MS): suitable
solubility
H2O: soluble 10 mg/mL
UniProt accession no.
storage temp.
−20°C
Gene Information
bovine ... TRYP8(282603)
Looking for similar products? Visit Product Comparison Guide
General description
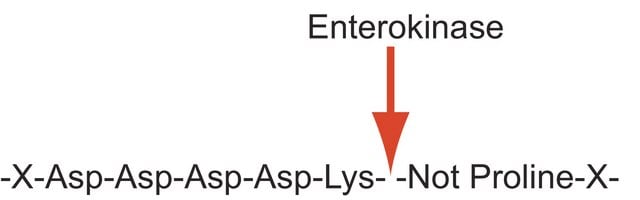
Application
- the secondary structure analysis of proteins in H2O solution using single-pass attenuated total reflection Fourier transform infrared (ATR-FT-IR) microscopy
- tuning and calibration of electrospray ionization quadrupole time-of-flight (ESI-Q-TOF) mass spectrometer
- the secondary structure analysis of proteins by infrared (IR) spectroscopy
- SDS-PAGE as a molecular weight standard (24kDa)
Biochem/physiol Actions
Unit Definition
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service