D9904
Nα,Nε-Diacetyl-Lys-D-Ala-D-Ala
carboxypeptidase substrate
Synonym(s):
(Ac)2-L-Lys-D-Ala-D-Ala
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H28N4O6
CAS Number:
Molecular Weight:
372.42
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
composition
Peptide content, ≥85%
solubility
water: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
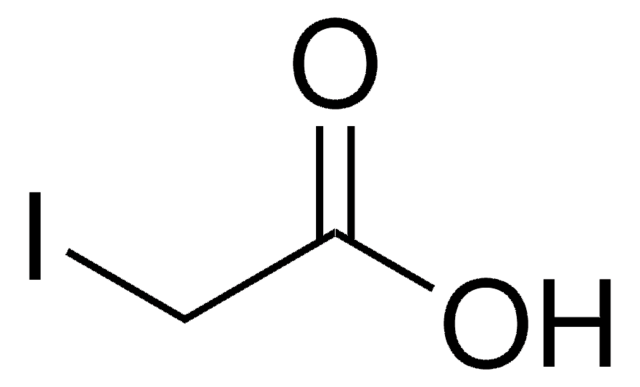
OC([C@@H](C)NC([C@@H](C)NC([C@@H](NC(C)=O)CCCCNC(C)=O)=O)=O)=O
InChI
1S/C16H28N4O6/c1-9(14(23)19-10(2)16(25)26)18-15(24)13(20-12(4)22)7-5-6-8-17-11(3)21/h9-10,13H,5-8H2,1-4H3,(H,17,21)(H,18,24)(H,19,23)(H,20,22)(H,25,26)
InChI key
VIHGYLJIMMKSBR-UHFFFAOYSA-N
Substrates
Substrate for penicillin-sensitive D-alanine carboxypeptidase.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cleidiane G Zampronio et al.
Analytical chemistry, 76(17), 5172-5179 (2004-09-18)
Electrospray ionization (ESI) is extensively used in the analysis of biological compounds; yet some fundamental properties of this technique are not completely understood. It is widely recognized that care should be exercised when noncovalent complexes are being studied by ESI
T R Herrin et al.
Journal of medicinal chemistry, 28(9), 1371-1375 (1985-09-01)
A series of ristocetin analogues with modifications (OH, C=O, C=NOH, NCOCH3) at the C-1' amino group was synthesized and found to possess antibacterial activity against gram-positive bacteria and to bind to Ac2-Lys-D-Ala-D-Ala, a model for the antibiotic's site of action.
M Leyh-Bouille et al.
The Biochemical journal, 235(1), 177-182 (1986-04-01)
The values of the kinetic parameters that govern the interactions between the Streptomyces K15 DD-peptidase and beta-lactam compounds were determined by measuring the inactivating effect that these compounds exert on the transpeptidase activity of the enzyme and, in the case
Zhibo Yang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(9), 2081-2090 (2009-01-22)
Charge matters! The charge state significantly influences the conformation and the binding energy between vancomycin antibiotic and bacterial cell-wall analogue peptides (see figure). Surface-induced dissociation (SID) studies provide a quantitative comparison between the stabilities of different charge states of the
M Nguyen-Distèche et al.
The Biochemical journal, 207(1), 109-115 (1982-10-01)
The membrane-bound, 26 000-Mr penicillin-binding protein of Streptomyces K15 has been isolated in the form of an effective, penicillin-sensitive D-alanyl-D-alanine-cleaving peptidase exhibiting high transpeptidase activity (greater than 95%) and very low carboxy-peptidase activity (less than 5%). The penicillin-binding protein/transpeptidase can
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service