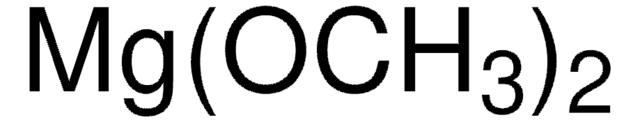292788
Potassium methoxide
95%
Synonym(s):
Potassium methylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3OK
CAS Number:
Molecular Weight:
70.13
Beilstein:
3551544
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
SMILES string
[K+].C[O-]
InChI
1S/CH3O.K/c1-2;/h1H3;/q-1;+1
InChI key
BDAWXSQJJCIFIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Potassium methoxide is a basic nucleophile used in the dehalogenation of arylhalides.
Application
Potassium methoxide can be used:
- As a methoxylating agent for methoxylation of bromo- and mesyloxyalkanes in the presence of an ionic liquid [bmim][BF4].
- As a catalyst for the synthesis of dimethyl carbonate and methyl formate.
- As a catalyst for the transesterification of sunflower oil for the production of biodiesel.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-heat. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 2
Flash Point(F)
51.8 °F - closed cup
Flash Point(C)
11 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The effect of Mo(CO)6 as a co-catalyst in the carbonylation of methanol to methyl formate catalyzed by potassium methoxide under CO, syngas and H2 atmospheres. HP-IR observation of the methoxycarbonyl intermediate of Mo(CO)6.
Jali S, et al.
J. Mol. Catal. A: Chem., 348(1-2), 63-69 (2011)
Oligoethylene Glycols as Highly Efficient Mutifunctional Promoters for Nucleophilic-Substitution Reactions
Vinod J H et al.
Chemistry?A European Journal , 18, 3918-3924 (2012)
General and practical potassium methoxide/disilane-mediated dehalogenative deuteration of (hetero) arylhalides
Xin W et al.
Journal of the American Chemical Society, 140, 10970-10974 (2018)
Synthesis of dimethyl carbonate from methanol and carbon dioxide using potassium methoxide as catalyst under mild conditions.
Cai Q, et al.
Catalysis Letters, 103(3-4), 225-228 (2005)
Significantly enhanced reactivities of the nucleophilic substitution reactions in ionic liquid.
Kim DW, et al.
The Journal of Organic Chemistry, 68(11), 4281-4285 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service