About This Item
Recommended Products
Quality Level
Assay
≥95%
form
powder
UniProt accession no.
storage temp.
−20°C
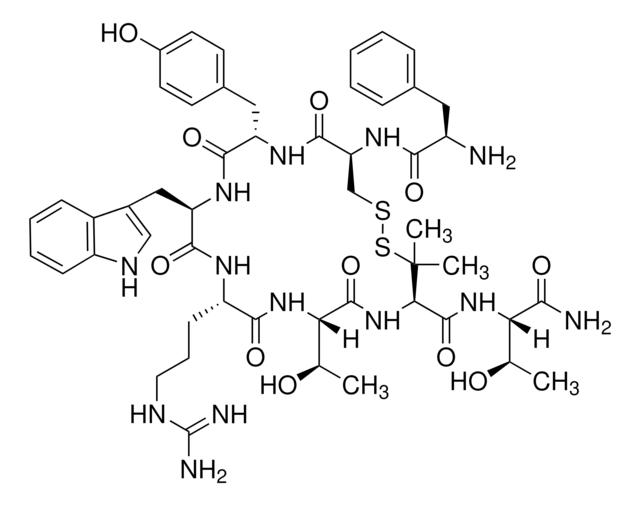
SMILES string
C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H](N)Cc5ccccc5)[C@@H](C)O)C(N)=O
InChI
1S/C51H69N13O11S2/c1-26(65)39(42(53)68)62-49(75)41-51(3,4)77-76-25-38(61-43(69)33(52)21-28-11-6-5-7-12-28)47(73)59-36(22-29-16-18-31(67)19-17-29)45(71)60-37(23-30-24-57-34-14-9-8-13-32(30)34)46(72)58-35(15-10-20-56-50(54)55)44(70)63-40(27(2)66)48(74)64-41/h5-9,11-14,16-19,24,26-27,33,35-41,57,65-67H,10,15,20-23,25,52H2,1-4H3,(H2,53,68)(H,58,72)(H,59,73)(H,60,71)(H,61,69)(H,62,75)(H,63,70)(H,64,74)(H4,54,55,56)/t26-,27-,33-,35+,36+,37-,38+,39+,40+,41-/m1/s1
InChI key
OFMQLVRLOGHAJI-FGHAYEPSSA-N
Gene Information
rat ... Pnoc(25516)
Amino Acid Sequence
Application
- to study the anti-hyperalgesic effect of dipeptidyl peptidase 4 (DPP4) inhibitor isoleucine-proline-isoleucine (IPI) and vildagliptin in carrageenan-induced inflammation
- to study the role of MOR in glutamate and gamma-aminobutyric acid (GABA) efflux during predator stress in rats
- to determine the endogenous opioid peptide involved in blocking pain induced by activated gastrin-releasing peptide (Grp+) neurons
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service