E35400
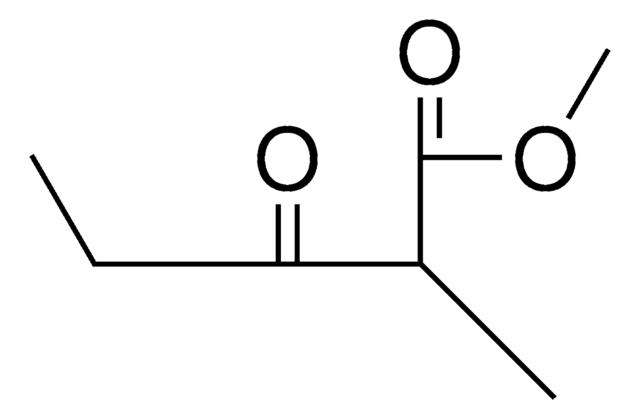
Ethyl 2-methylacetoacetate
90%
Synonym(s):
Ethyl 2-methyl-3-oxobutanoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3COCH(CH3)CO2C2H5
CAS Number:
Molecular Weight:
144.17
Beilstein:
1071742
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
90%
form
liquid
refractive index
n20/D 1.418 (lit.)
bp
187 °C (lit.)
density
1.019 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(C)C(C)=O
InChI
1S/C7H12O3/c1-4-10-7(9)5(2)6(3)8/h5H,4H2,1-3H3
InChI key
FNENWZWNOPCZGK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Ethyl 2-methylacetoacetate is used as a substrate in the rhenium-catalyzed synthesis of multisubstituted aromatic compounds.
- It can be employed in the synthesis of coumarin derivatives via Pechmann condensation.
- It undergoes dehydration to yield conjugated alkynyl and allenyl esters.
- It is also used in the total synthesis of chlorotonil A, yangjinhualine A, (+)- and (−)-saudin.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of coumarins catalyzed by eco-friendly W/ZrO2 solid acid catalyst.
Reddy B M, et al.
Synthetic Communications, 31(23), 3603-3607 (2001)
Selective One-Pot Synthesis of Allenyl and Alkynyl Esters from β-Ketoesters.
Maity P and Lepore S D
The Journal of Organic Chemistry, 74(1), 158-162 (2008)
An enantioselective total synthesis of (+)-and (−)-saudin. Determination of the absolute configuration.
Boeckman R K, et al.
Journal of the American Chemical Society, 124(2), 190-191 (2002)
Concise, regiocontrolled synthesis of yangjinhualine A.
Boukouvalas J and McCann L C
Tetrahedron Letters, 52(11), 1202-1204 (2011)
Michal Plž et al.
Molecules (Basel, Switzerland), 25(18) (2020-09-24)
The co-immobilization of ketoreductase (KRED) and glucose dehydrogenase (GDH) on highly cross-linked agarose (sepharose) was studied. Immobilization of these two enzymes was performed via affinity interaction between His-tagged enzymes (six histidine residues on the N-terminus of the protein) and agarose
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service