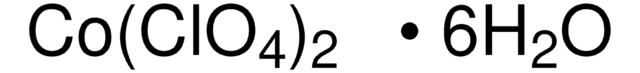401439
Zinc perchlorate hexahydrate
Synonym(s):
Perchloric acid zinc hydrate
About This Item
Recommended Products
form
crystals and lumps
Quality Level
reaction suitability
reagent type: oxidant
SMILES string
O.O.O.O.O.O.[Zn++].[O-]Cl(=O)(=O)=O.[O-]Cl(=O)(=O)=O
InChI
1S/2ClHO4.6H2O.Zn/c2*2-1(3,4)5;;;;;;;/h2*(H,2,3,4,5);6*1H2;/q;;;;;;;;+2/p-2
InChI key
PADPILQDYPIHQQ-UHFFFAOYSA-L
General description
Application
- For acylation of electron-deficient phenols, sterically hindered alcohols and amines.
- For the synthesis of 2-hydroxy sulfides by the opening of epoxide rings with thiols.
- For solvent‐free condensation of carboxylic acids with alcohols.
- An electrolyte for aqueous Zinc batteries.
- A precursor for the synthesis of highly fluorescent ZnSe quantumdots.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Ox. Sol. 2 - Skin Corr. 1B
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Colloidal quantum dots (CQDs) are semiconducting crystals of only a few nanometers (ca. 2–12 nm) coated with ligand/surfactant molecules to help prevent agglomeration.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)