05-915
Anti-N-Cadherin Antibody, clone 13A9
culture supernatant, clone 13A9, Upstate®
Synonym(s):
Cadherin-2, CD325, CDw325, N-cadherin, Neural cadherin
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
culture supernatant
antibody product type
primary antibodies
clone
13A9, monoclonal
species reactivity
human
packaging
antibody small pack of 25 μL
manufacturer/tradename
Upstate®
technique(s)
immunocytochemistry: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
NCBI accession no.
UniProt accession no.
shipped in
ambient
target post-translational modification
unmodified
Gene Information
human ... CDH2(1000)
Related Categories
General description
Specificity
Immunogen
Application
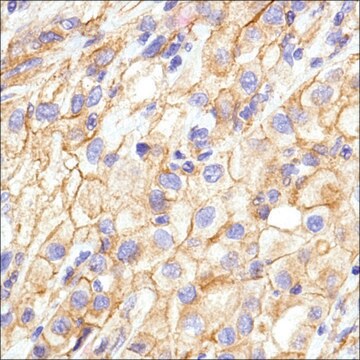
Immunohistochemistry Analysis: A representative lot detected strong N-cadherin immunoreactivity in paraffin-embedded rectal cancer (RC) tissues with positive regional lymph node metastasis (RLNM) status, while only weak N-cadherin immunoreactivity was detected in RC with negative RLNM, and no N-cadherin staining was seen in normal colorectal epithelium (Fan, X.J., et al. (2012). Br. J. Cancer. 106(11):1735-1741).
Immunohistochemistry Analysis: A representative lot detected N-cadherin immunoreactivity in formalin-fixed, paraffin-embedded hepatocellular carcinoma (HCC) tissue sections. A significant inverse correlation was found between RUNX3 and N-cadherin expression levels (Tanaka, S., et al. (2012). Int. J. Cancer. 131(11):2537-2546).
Western Blotting Analysis: A representative lot detected an upregulated N-cadherin expression in CCL185 carcinoma cells following transient Epstein-Barr virus (EBV) infection. The EMT-like phenotype remained even after viral loss by culture selection pressure withdrawal (Queen, K.J., et al. (2013). Int. J. Cancer. 132(9):2076-2086).
Western Blotting Analysis: A representative lot detected N-cadherin in Hep3B, Huh7, HLF and SK-Hep1 human hepatocellular carcinoma (HCC) cell lysates (Tanaka, S., et al. (2012). Int. J. Cancer. 131(11):2537-2546).
Western Blotting Analysis: A representative lot detected both the unprocessed (pro-) and processed (mature) forms of N-cadherin in HeLa cell lysate (Wahl, J.K. 3rd., et al. (2003). J. Biol. Chem. 278(19):17269-17276).
Western Blotting Analysis: A representative lot detected N-cadherin in WI-38 human fibroblast lysate, but not in JAr human placental choriocarcinoma cell lysate (Knudsen, K.A., et al. (1995). J. Cell Biol. 130(1):67-77).
Immunocytochemistry Analysis: A representative lot detected N-cadherin immunoreactivity localized primarily at the cell-cell borders by fluorescent immunocytochemistry staining of 1% paraformaldehyde-fixed, methanol-permeabilized HeLa cells (Wahl, J.K. 3rd., et al. (2003). J. Biol. Chem. 278(19):17269-17276).
Immunocytochemistry Analysis: A representative lot detected N-cadherin immunoreactivity colocalized with those of alpha- and beta-catenin by dual fluorescent immunocytochemistry staining of fixed WI-38 human fibroblasts (Knudsen, K.A., et al. (1995). J. Cell Biol. 130(1):67-77).
Immunoprecipitation Analysis: Representative lots co-immunoprecipitated alpha-catenin, beta-catenin, and plakoglobin with N-cadherin from WI-38 human fibroblast and HeLa cell lysates (Wahl, J.K. 3rd., et al. (2003). J. Biol. Chem. 278(19):17269-17276; Knudsen, K.A., et al. (1995). J. Cell Biol. 130(1):67-77).
Cell Structure
Adhesion (CAMs)
Quality
Western Blotting Analysis: A 1:1000-5000 dilution of this hybridoma culture supernatant detected N-cadherin in HeLa cell lysate.
Target description
Linkage
Physical form
Storage and Stability
Analysis Note
HeLa cell lysate
Legal Information
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
recommended
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service