LM4103
Avanti
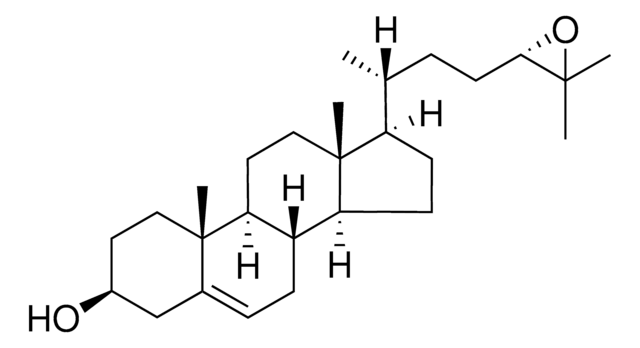
7α-hydroxycholesterol (D7)
Avanti Research™ - A Croda Brand LM4103, methanol solution
Synonym(s):
cholest-5-en-3β,7α-diol(d7)
About This Item
Recommended Products
form
methanol solution
packaging
pkg of 1 × 1 mL (LM4103-1EA)
manufacturer/tradename
Avanti Research™ - A Croda Brand LM4103
concentration
~10 μg/mL (Refer to C of A for lot specific concentration. )
shipped in
dry ice
storage temp.
−20°C
SMILES string
[H][C@@]12[C@]([C@](CC[C@H](O)C3)(C)C3=C[C@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCCC([2H])(C([2H])([2H])[2H])C([2H])([2H])[2H]
General description
Application
- as a deuterated sterol standard for the extraction of lipids from cultured cells
- in liquid chromatography-high-resolution mass spectrometry method (LC-MS/HR-MS) for the quantification
- to synthesize d4-7 -hydroxy-3-oxo-4-cholestenoic acid (d4-7-HOCA)
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service