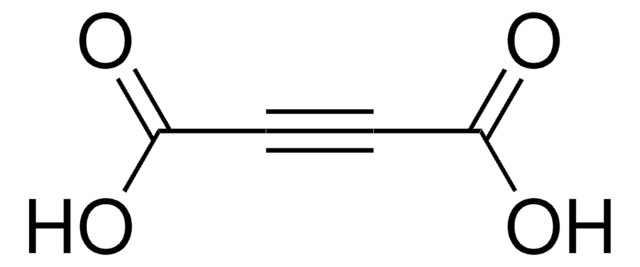A15401
Acetylenedicarboxylic acid monopotassium salt
≥98%
Synonym(s):
mono-Potassium 2-butynedioate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOOCC≡CCOOK
CAS Number:
Molecular Weight:
152.15
Beilstein:
3571639
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
powder
SMILES string
[K+].OC(=O)C#CC([O-])=O
InChI
1S/C4H2O4.K/c5-3(6)1-2-4(7)8;/h(H,5,6)(H,7,8);/q;+1/p-1
InChI key
KLLYWRUTRAFSJT-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Identification of an intermediate in the de novo formation of nicotinamide adenine dinucleotide in Escherichia coli.
J T Heard et al.
Biochemical and biophysical research communications, 95(4), 1517-1521 (1980-08-29)
Lothar Brecker et al.
European journal of biochemistry, 270(7), 1393-1398 (2003-03-26)
Only 2% of the known natural products with acetylenic bonds are alpha-alkynoates. Their polarized, conjugated triple bond is an optimal target for an enzymic hydration. Therefore they are good substrates for the enzymes involved in metabolism of acetylenic compounds, resulting
Heiner Eckert
Molecules (Basel, Switzerland), 17(1), 1074-1102 (2012-01-24)
A collection of smart multicomponent reactions (MCRs) with continuative post condensation cyclizations (PCCs) is presented to construct conventional three- to seven-membered heterocyclic compounds in diversity oriented syntheses (DOS). These will provide a high degree of applying economical and ecological advantages
Issa Yavari et al.
Molecular diversity, 10(2), 265-270 (2006-06-17)
Protonation of the reactive intermediates produced in the reaction between alkyl(aryl) isocyanides and dialkyl acetylenedicarboxylates by indan-1,3-dione leads to vinylnitrilium cations, which undergo carbon centered Michael type addition with the conjugate base of the CH-acid to produce functionalized 5-oxo-4,5-dihydroindeno[1,2-b]pyrans.
A S Subbaraman et al.
Origins of life, 10(4), 343-347 (1980-12-01)
A number of routes have been suggested for the prebiotic synthesis of uracil involving the reaction of urea with malic acid, propiolic acid, cyanoacetylene and others. Cyanoacetylene has been detected in the interstellar medium as well as simulated prebiotic experiments.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service