All Photos(1)
About This Item
Linear Formula:
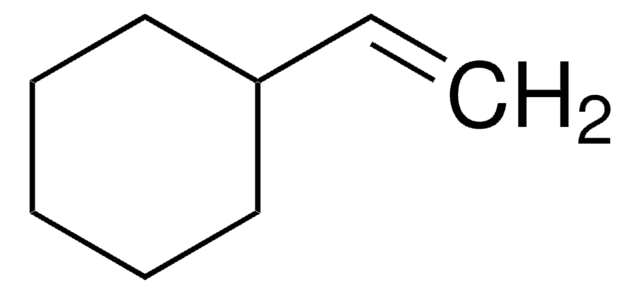
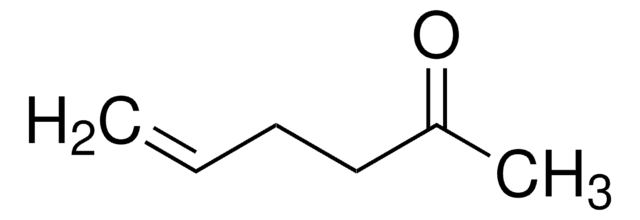
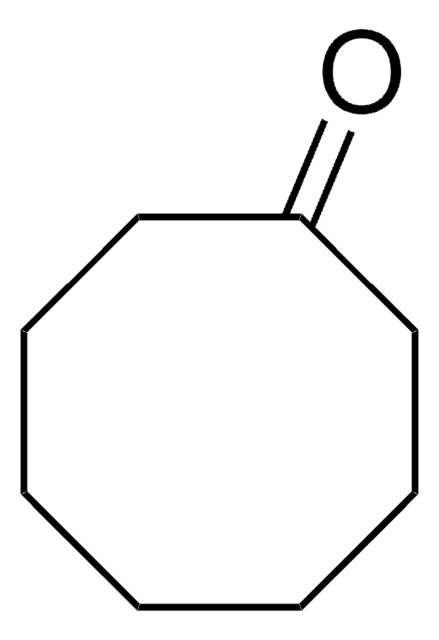
H2C=CHCH2C6H9(=O)
CAS Number:
Molecular Weight:
138.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
94 °C/23 mmHg (lit.)
density
0.927 g/mL at 25 °C (lit.)
functional group
allyl
ketone
SMILES string
C=CCC1CCCCC1=O
InChI
1S/C9H14O/c1-2-5-8-6-3-4-7-9(8)10/h2,8H,1,3-7H2
InChI key
UPGHEUSRLZSXAE-UHFFFAOYSA-N
General description
2-Allylcyclohexanone is a β,γ-unsaturated ketone. Selective oxidation of 2-allylcyclohexanone by benzonitrile-hydrogen peroxide has been reported. Asymmetric synthesis of 2-alkylcyclohexanones by alkylation of cyclohexanone enamines (III) of L-proline ester derivatives has been described. Oxidation of 2-allylcyclohexanone by 90% hydrogen peroxide catalyzed by arsonated polystyrene has been studied.
Application
2-Allylcyclohexanone may be used in the synthesis of the following:
- bicyclo[3.3.1]non-2-en-9-one
- R-(-)-epilachnene, an antipode of the defensive droplets from the Mexican bean beetle, Epilachna varivestis
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Modified Thermodynamically Controlled Deracemization of 2-Allylcyclohexanone and Its Application to Asymmetric Synthesis of (R)-(-)-Epilachnene.
Kaku H, et al.
Chemistry Letters (Jpn), 33(5), 516-517 (2004)
Biphase and triphase catalysis. Arsonated polystyrenes as catalysts in the Baeyer-Villiger oxidation of ketones by aqueous hydrogen peroxide.
Jacobson SE, et al.
Journal of the American Chemical Society, 101(23), 6938-6946 (1979)
Bridget McCarthy Cole et al.
The Journal of organic chemistry, 61(22), 7832-7847 (1996-11-01)
Mn(III)-based oxidative free-radical cyclization of unsaturated ketones is a versatile synthetic procedure with broad applicability. For example, oxidation of cyclopentanone 1a with 2 equiv of Mn(OAc)(3).2H(2)O and 1 equiv of Cu(OAc)(2).H(2)O in AcOH at 80 degrees C for 1.5 h
Stereochemical Studies. IX. Asymmetric Synthesis of 2-Alkylcyclo-hexanones with Enamine Alkylation.
Hiroi K, et al.
Chemical & Pharmaceutical Bulletin, 20, 246-257 (1972)
A simplified procedure for epoxidation by benzonitrile-hydrogen peroxide. Selective oxidation of 2-allylcyclohexanone.
Payne GB.
Tetrahedron, 18(6), 763-765 (1962)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service