376914
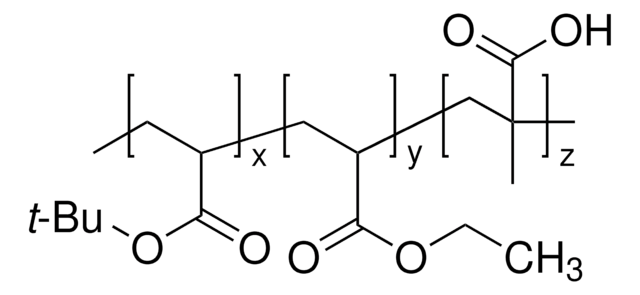
Poly(methyl methacrylate-co-methacrylic acid)
average Mw ~34,000 by GPC, average Mn ~15,000 by GPC
Synonym(s):
Poly(methacrylic acid-co-methyl methacrylate)
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
feed ratio
1:0.016 (methyl methacrylate:methacrylic acid)
mol wt
average Mn ~15,000 by GPC
average Mw ~34,000 by GPC
inherent viscosity
0.19 dL/g(lit.)
transition temp
Tg 105 °C
SMILES string
O(C)C(=O)C(=C)C.OC(=O)C(=C)C
InChI
1S/C5H8O2.C4H6O2/c1-4(2)5(6)7-3;1-3(2)4(5)6/h1H2,2-3H3;1H2,2H3,(H,5,6)
InChI key
IWVKTOUOPHGZRX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Ultrafine Nanoparticles of Poly(Methyl Methacrylate-co-Methacrylic Acid) Loaded with Aspirin: This study focuses on the preparation and potential therapeutic applications of biocompatible nanoparticles loaded with aspirin, highlighting their suitability for medical use (López-Muñoz et al., 2019).
- Biocompatible and biodegradable ultrafine nanoparticles of poly (methyl methacrylate-co-methacrylic acid) prepared via semicontinuous heterophase: Discusses the synthesis of biodegradable nanoparticles for potential biomedical applications, emphasizing their eco-friendly nature and suitability for drug delivery systems (Saade et al., 2016).
- Synthesis and characteristics of poly(methyl methacrylate‐co‐methacrylic acid)/poly(methacrylic acid‐co‐N‐isopropylacrylamide) thermosensitive semi‐hollow latex: Explores the unique properties of thermosensitive latex particles for advanced material applications, potentially useful in smart drug delivery systems (Lee et al., 2014).
- Preparation and release behavior of poly(methyl methacrylate-co-methacrylic acid)-based electrospun nanofibrous mats loaded with doxorubicin: Examines the use of polymeric nanofibers for controlled drug release, particularly for cancer treatment, demonstrating the versatility of poly(methyl methacrylate-co-methacrylic acid) in pharmaceutical applications (López-Muñoz et al., 2023).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kateřina Dvořáčková et al.
Medicina (Kaunas, Lithuania), 48(4), 192-202 (2012-07-28)
Eudragit® NM was investigated as a matrix former in combination with microcrystalline cellulose as an insoluble filler for preparing controlled-release tablets containing model drugs with different solubility. Three sets of matrix tablets differing in the drug-to-filler ratio (1:1, 2:1, and
Katia P Seremeta et al.
Colloids and surfaces. B, Biointerfaces, 102, 441-449 (2012-09-27)
The design of simple and scalable drug delivery systems to target the central nervous system (CNS) could represent a breakthrough in the addressment of the HIV-associated neuropathogenesis. The intranasal (i.n.) route represents a minimally invasive strategy to surpass the blood-brain
Rouslan I Moustafine et al.
International journal of pharmaceutics, 439(1-2), 17-21 (2012-10-09)
Interpolymer interactions between the countercharged methacrylate copolymers Eudragit(®) RL 30D (polycation) and Eudragit(®) FS 30D (polyanion), were investigated in conditions mimicking the gastrointestinal environment. The formation of inter-macromolecular ionic bonds between Eudragit(®) RL 30D and Eudragit(®) FS 30D was investigated
Martins Emeje et al.
Acta pharmaceutica (Zagreb, Croatia), 62(1), 71-82 (2012-04-05)
Sustained release (SR) dosage forms enable prolonged and continuous deposition of the drug in the gastrointestinal (GI) tract and improve the bioavailability of medications characterized by a narrow absorption window. In this study, a new strategy is proposed for the
Pravin K Pawar et al.
Acta pharmaceutica (Zagreb, Croatia), 62(1), 93-104 (2012-04-05)
The objective of the present investigation was to prepare and evaluate ocular inserts of moxifloxacin. An ocular insert was made from an aqueous dispersion of moxifloxacin, sodium alginate, polyvinyl alcohol, and dibutyl phthalate by the film casting method. The ocular
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service