All Photos(1)
About This Item
Empirical Formula (Hill Notation):
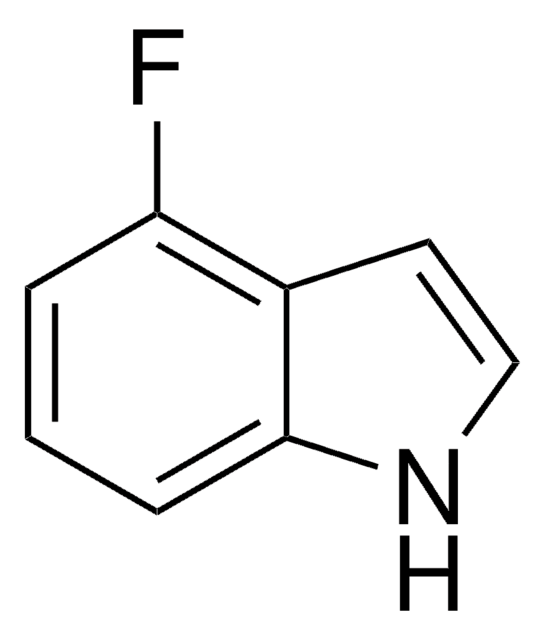
C8H6FN
CAS Number:
Molecular Weight:
135.14
Beilstein:
112192
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
72-76 °C (lit.)
functional group
fluoro
SMILES string
Fc1ccc2cc[nH]c2c1
InChI
1S/C8H6FN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChI key
YYFFEPUCAKVRJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Fluoroindole is a halogen substituted indole. Experimental ionization potential of 6-fluoroindole has been evaluated. Preparation of 6-fluoroindole via nitration of indoline has been reported.
Application
6-Fluoroindole may be used as reactant in the preparation of:
- tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- antibacterial agents
- antifungal agents
- Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the management of hyperglycemia in diabetes
- potent selective serotonin reuptake inhibitors
- inhibitors of HIV-1 attachment
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 5-and 6-Halogenoindoles from Indoline
Ikan R, et al.
Israel J. Chem., 2(2), 37-42 (1964)
Journal of Medicinal Chemistry, 36, 2242-2242 (1993)
Chun-Hsu Yao et al.
Journal of medicinal chemistry, 54(1), 166-178 (2010-12-07)
A novel series of N-linked β-D-xylosides were synthesized and evaluated for inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) in a cell-based assay. Of these, the 4-chloro-3-(4-cyclopropylbenzyl)-1-(β-D-xylopyranosyl)-1H-indole 19m was found to be the most potent inhibitor, with an EC(50) value
H Dalton King et al.
Journal of medicinal chemistry, 53(21), 7564-7572 (2010-10-19)
A series of conformationally restricted homotryptamines has been synthesized and shown to be potent inhibitors of hSERT. Conformational restriction of the homotryptamine side chain was attained by the insertion of a cyclopentyl ring, with the indole ring and the terminal
Na, Y. M.
Bull. Korean Chem. Soc., 31, 3467-3467 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service