188344
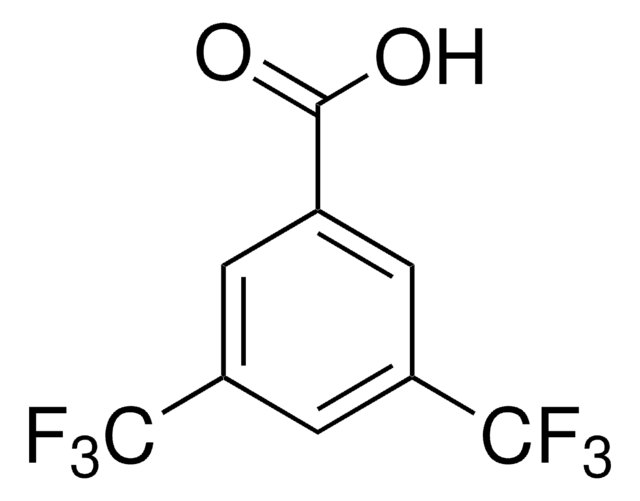
3-(Trifluoromethyl)benzoic acid
99%
Synonym(s):
α,α,α-Trifluoro-m-toluic acid, 3-Carboxybenzotrifluoride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3C6H4CO2H
CAS Number:
Molecular Weight:
190.12
Beilstein:
2049239
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
238.5 °C/775 mmHg (lit.)
mp
104-106 °C (lit.)
functional group
carboxylic acid
fluoro
SMILES string
OC(=O)c1cccc(c1)C(F)(F)F
InChI
1S/C8H5F3O2/c9-8(10,11)6-3-1-2-5(4-6)7(12)13/h1-4H,(H,12,13)
InChI key
FQXQBFUUVCDIRK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
pKa values of 3-(trifluoromethyl)benzoic acid in water and in methanol has been measured. Solubility of 3-(trifluoromethyl)benzoic acid in dense carbon dioxide was evaluated to investigate the influence of fluorination on the solubility of organic pharmaceuticals in dense carbon dioxide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B F Taylor et al.
FEMS microbiology letters, 110(2), 213-216 (1993-06-15)
m- and p-trifluoromethyl (TFM)-benzoates are incompletely degraded by aerobic bacteria that catabolize alkylbenzoates; biodegradation ceases after ring-fission with the accumulation of a trifluoromethyl muconate semialdehyde (2-hydroxy-6-oxo-7,7,7-trifluorohepta-2,4-dienoate, TFHOD) which is resistant to biochemical attack. A bacterium (Strain V-1), isolated from sea-water
Aneela Maalik et al.
Bioorganic chemistry, 88, 102946-102946 (2019-05-06)
An irrefutable advancement has been noted for the infectious diseases caused due to ureolytic bacteria through the development of various drugs. Keeping in mind the extremely valuable synthetic utility and medicinal significance of thiourea derivatives, synthesis of new 3-trifluoromethyl benzoic
Solubility of fluorinated pharmaceuticals in dense carbon dioxide.
Laitinen A, et al.
Organic Process Research & Development, 4(5), 353-356 (2000)
K H Engesser et al.
Archives of microbiology, 149(3), 188-197 (1988-01-01)
The TOL plasmid-encoded enzymes of the methylbenzoate pathway in Pseudomonas putida mt-2 cometabolized 3-trifluoromethyl (TFM)-benzoate. Two products, 3-TFM-1,2-dihydroxy-2-hydrobenzoate (3-TFM-DHB) and 2-hydroxy-6-oxo-7,7,7-trifluoro-hepta-2,4-dienoate (7-TFHOD) were identified chemically and by spectroscopic properties. TFM-substituted analogues of the metabolites of the methylbenzoate pathway were generally
S A Selifonov et al.
Biochemical and biophysical research communications, 213(3), 759-767 (1995-08-24)
Pseudomonas aeruginosa strain 142 oxidizes 2-halobenzoates via a multicomponent oxygenase (V. Romanov and R.P. Hausinger, J. Bacteriol., 1994, 176(11), 3368-3374). The intermediacy of a highly unstable cis-diol in the reaction has been proposed. Direct evidence for this is currently lacking
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service