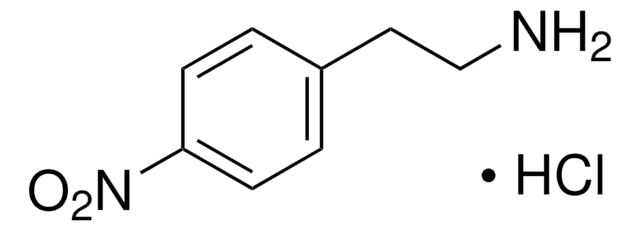
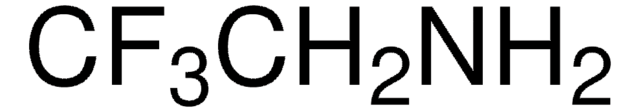180386
2,2,2-Trifluoroethylamine hydrochloride
98%
Synonym(s):
2-Amino-1,1,1-trifluoroethane hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CF3CH2NH2 · HCl
CAS Number:
Molecular Weight:
135.52
Beilstein:
3652103
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
220-222 °C (subl.) (lit.)
functional group
amine
fluoro
SMILES string
Cl.NCC(F)(F)F
InChI
1S/C2H4F3N.ClH/c3-2(4,5)1-6;/h1,6H2;1H
InChI key
ZTUJDPKOHPKRMO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,2,2-Trifluoroethylamine hydrochloride was used in the derivatization of aqueous carboxylic acids to the corresponding 2,2,2-trifluoroethylamide derivative.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Quincy LaRon Ford et al.
Journal of chromatography. A, 1145(1-2), 241-245 (2007-02-20)
We report a technique for the rapid, room temperature derivatization of aqueous carboxylic acids to the corresponding 2,2,2-trifluoroethylamide derivative. 3-Ethyl-1-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDC) and 2,2,2-trifluoroethylamine hydrochloride (TFEA) were added to aqueous samples of several acids of interest in environmental analytical chemistry
Yoshikazu Hattori et al.
Journal of biomolecular NMR, 68(4), 271-279 (2017-08-02)
The preparation of stable isotope-labeled proteins is important for NMR studies, however, it is often hampered in the case of eukaryotic proteins which are not readily expressed in Escherichia coli. Such proteins are often conveniently investigated following post-expression chemical isotope
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service