115312
N-ω-Methyltryptamine
99%
Synonym(s):
2-(Indol-3-yl)-N-methylethanamine, 3-(2-Methylaminoethyl)indole, 3-(2-[Methylamino]ethyl)indole, N-Monomethyltryptamine, Dipterine, N10-Methyltryptamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H14N2
CAS Number:
Molecular Weight:
174.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
87-89 °C (lit.)
functional group
amine
SMILES string
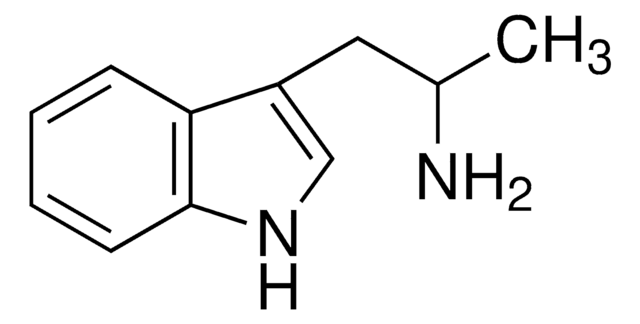
CNCCc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-12-7-6-9-8-13-11-5-3-2-4-10(9)11/h2-5,8,12-13H,6-7H2,1H3
InChI key
NCIKQJBVUNUXLW-UHFFFAOYSA-N
Application
N-ω-Methyltryptamine was used in the preparation of N-acetyl-α−methyltryptamine.
N-ω-methyltryptamine was used in the biosynthesis of dolichantoside using U. tomentosa protein extracts.
Reactant for preparation of:
- Manzamine analogues for the control of neuroinflammation and cerebral infections
- Serotonin 4 receptors (5-HT4) receptor agonists
- A sulful-containing indole alkaloid, glypetelotine
- Selective inhibitors of cyclin dependent kinase (CDK4)
- Antagonist of the human tachykinin NK-2 receptor
- Inhibitors of the tyrosine-specific protein kinase pp60c-src SH2 Domain
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T J Williams et al.
European journal of pharmacology, 245(3), 197-201 (1993-05-15)
The binding of [3H]5-hydroxytryptamine (5-HT) to rat enteric membranes was inhibited by the inclusion of 5-HT 2-methyl-5-HT, 5-hydroxytryptophan, N,N,N-triethyltryptamine and 2-Br-N,N-diethyltryptamine in the incubation buffer. In contrast, tryptamine, 5-methoxytryptamine and 2-methyl-N,N-diethyltryptamine enhanced binding. Ascorbate and dithiothreitol facilitated and reduced binding
R W Walker et al.
Journal of chromatography, 289, 223-229 (1984-04-27)
A capillary column gas-liquid chromatography selected ion monitoring-based method was developed for the measurement of [13C,15N]N-methyltryptamine ( NMT ) in human urine. The method was employed to establish the extent of conversion of [13C,15N]tryptamine to the correspondingly labeled NMT in
Strictosidine-related enzymes involved in the alkaloid biosynthesis of Uncaria tomentosa root cultures grown under oxidative stress.
Vera-Reyes I, Huerta-Heredia AA, Ponce-Noyola T, et al.
Biotechnology Progress, doi:10-doi:10 (2013)
Luigi Servillo et al.
Journal of agricultural and food chemistry, 60(37), 9512-9518 (2012-09-11)
The occurrence of N-methylated tryptamine derivatives in bergamot plant (Citrus bergamia Risso et Poit) is reported for the first time. Interestingly, the most abundant of these substances is N,N,N-trimethyltryptamine, which has not been previously identified in any citrus plant. The
R N Cory et al.
The Journal of pharmacology and experimental therapeutics, 236(1), 48-54 (1986-01-01)
The contractile response of the isolated rabbit aorta elicited by 5-hydroxytryptamine (5-HT) and five partial agonists acting on the 5-HT2 receptor were separated into a phasic and a tonic response by altering the [Ca++] in the buffer. A kinetic analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)