517933
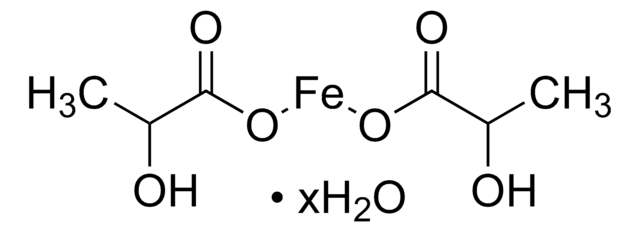
Iron(II) acetate
≥99.99% trace metals basis
Synonym(s):
Ferrous acetate, Iron acetate [Fe(OAc)2 ], Iron diacetate
About This Item
Recommended Products
assay
≥99.99% trace metals basis
form
solid
reaction suitability
core: iron
mp
190-200 °C (dec.) (lit.)
SMILES string
CC(=O)O[Fe]OC(C)=O
InChI
1S/2C2H4O2.Fe/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
LNOZJRCUHSPCDZ-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A precursor for synthesizing iron oxide and iron-based nanostructures which are employed as anode materials for lithium-ion batteries and supercapacitors.
- A precursor in the synthesis of iron oxide nanoparticles. These particles are incorporated into carbon nanofibers for use in supercapacitor applications.
- A precursor to synthesize hematite nanoparticles for applications in solar cells. These nanoparticles exhibit shape-dependent optical properties and can be used for imaging, photocatalysis, and solar cells. The product was used to synthesize iron oxide nanoparticles which was further used to form iron oxide-poly(ethylene glycol) core-shell nanoparticles (NPs). The core-shell NPs were studied for self-assembly at liquid–liquid interfaces (SALI) forming monolayers.
Packaging
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Prof. Randal Lee discusses iron oxide magnetic nanospheres and nanocubes design considerations for biosensing applications.
The properties of many devices are limited by the intrinsic properties of the materials that compose them.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service