Polynuclear Aromatic Hydrocarbon Analysis in Olive Oil
Jack Wang
R&D APAC lab, Shanghai, China
Abstract
In this work, a gas chromatography-tandem mass spectrometry (GC-MS/MS) method for the analysis of twelve polycyclic aromatic hydrocarbons (PAHs) in olive oil is described. Two solid-phase extraction (SPE) methods were used for the clean-up of the oil samples, one with saponification following the GB 5009.265-2021 method, using a styrene/divinyl benzene resin (Supelclean™ ENVI Chrom P), and one simpler approach without, employing a multibed Supelclean™ EZ-POP NP SPE tube. Seven deuterium-labeled isotopic internal standards were utilized for the quantitative PAH analysis. The two sample preparation approaches were compared to each other. All results indicated the suitability of both methods for the determination of PAHs in olive oil meeting the GB acceptance criteria.
Section Overview
Introduction
Polycyclic Aromatic Hydrocarbons (PAHs) are pollutants of concern as they may have ecotoxicological effects, which can occur at all levels of the biological organization, from the molecular to the ecosystem level.1,2 The most potent carcinogenic PAH compounds include benzo[a]anthracene, benzo[a]pyrene and dibenz[a,h]anthracene.3,4 In the Chinese National Standard for Food Safety (GB Method 5009.265-2021) detailed analysis procedures for determination of PAHs are described: After saponification of a sample, clean-up using a styrene divinylbenzene copolymer solid-phase extraction (SPE) tube has to be performed, followed by GC-MS analysis.5 The first task of this study was to check the performance of the Supelclean™ ENVI™-Chrom P SPE tube, which is packed with a stationary phase requested by the GB 5009.265-2021 method, a styrene/divinyl benzene copolymer resin. The second set of experiments, referring to Stenerson et al., aimed to develop an alternative sample preparation approach for olive oil, omitting the delicate saponification step, by applying the Supelclean™ EZ-POP NP SPE with a simpler SPE procedure.6,7 This 2 bed SPE tube contains Florisil® (magnesium silicate hydrate) as the top sorbent bed and a mixture of zirconia- and C18-modified silica (Z-Sep/C18) as the bottom bed, to remove the matrix from the sample by chemical filtration (interference removal). In contrast to the Chinese National Standard, this work applied a more specific GC-MS/MS approach using a specialized ionic liquid GC PAH column (SLB®-ILPAH). The molecular structures of the twelve targeted PAHs are shown in Figure 1.
![Chemical structures of 12 PAHs Chemical structures of 12 polycyclic aromatic hydrocarbons (PAHs) arranged in a grid format on a white background. Each structure is drawn in black lines with clear labels below them, identifying the compounds. The top row includes (from left to right) Benzo[c]fluorene, Benz[a]anthracene, Chrysene, and Cyclopenta[c,d]pyrene, with their respective structures featuring fused aromatic rings in distinct arrangements. The second row includes (from left to right) 5-Methylchrysene, Benzo[b]fluoranthene, Benzo[k]fluoranthene, and Benzo[j]fluoranthene, with one structure displaying a methyl group. The third row features (from left to right) Benzo[a]pyrene, Dibenz[a,h]anthracene, Indeno[1,2,3-cd]pyrene, and Benzo[ghi]perylene, showcasing more complex fused-ring systems.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/food-and-beverage-testing-and-manufacturing/chemical-analysis/structure-12-pahs/structure-12-pahs.png)
Chemical structures of investigated PAHs.
Experimental
PAH Standard Preparation
- Diluent: Mix acetone and isooctane in a 1:1 (v/v) ratio until homogeneous to prepare the diluent.
- PAH stock solution: Prepare the PAH stock solution of twelve reference materials by dissolving appropriate amounts of the PAHs benzo[c]fluorene, benz[a]anthracene, chrysene, cyclopenta[cd]pyrene, 5-methylchrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[j]fluoranthene, benzo[a]pyrene, dibenz[a,h]anthracene, indeno[1,2,3-cd]pyrene and benzo[ghi]perylene) in diluent (c = 100 μg/mL).
- Deuterated PAH internal standard stock solution: Prepare the deuterated PAH internal standard stock solution of seven reference materials by dissolving appropriate amounts of the deuterated PAHs chrysene-d12, benz[a]anthracene-d12, benzo[b]fluoranthene-d12, benzo[a]pyrene-d12, dibenz[a,h]anthracene-d14, indeno[1,2,3-cd]pyrene-d12 and benzo[ghi]perylene-d12) in diluent (c = 100 μg/mL).
- PAH working solution (PAH WS): Transfer 50 μL of PAH stock solution into a 10 mL volumetric flask and top to mark with diluent to prepare the PAH working solution (c = 500 ng/mL).
- Deuterated PAH internal standard working solution (IS WS): Transfer 50 μL of deuterated PAH internal standard stock solution into a 10 mL volumetric flask and top to mark with diluent to prepare the deuterated PAH internal standard working solution (c = 500 ng/mL).
- PAH standard solutions (PAH SS): Combine 20, 40, 80, 100, 250, and 500 μL of PAH WS with 200 μL of IS WS in six separate 1.5 mL centrifuge tubes and make up to 1 mL with diluent. The final concentrations of PAHs in these solutions are 10, 20, 40, 50, 125, and 250 ng/mL, and the final concentration of all deuterated internal standards is 100 ng/mL.
Sample Preparation with Saponification and Supelclean™ ENVI™-Chrom P SPE Tube
An extra-virgin olive oil sample was purchased from a local supermarket and prepared according to the following procedure:
- Ethanolic solution of potassium hydroxide (1.5 M): Dissolve 8.40 g of potassium hydroxide in 100 mL of anhydrous ethanol using ultrasonication.
- Weigh 0.5 g (accurate to 0.001 g) of olive oil into a 15 mL centrifuge tube. For spiking experiments, pipette 10 μL of PAH WS to the oil sample and vortex for 30 seconds.
- Add 40 μL of IS WS to the sample and vortex for 30 seconds. Leave for five minutes prior to saponification.
- Add 5 mL of 1.5 M ethanolic potassium hydroxide solution and vortex for 30 seconds.
- For saponification, place the tube in a water bath at 70 ± 2 °C for 108 seconds.
- Cool to room temperature for five minutes using tap water (temperature 15 to 25°C).
- Add 4 mL water and 5 mL n-hexane, vortex for 2 minutes, then centrifuge at 10,000 rpm for 2 minutes.
- Subject the upper layer (hexane) of the extraction solution to SPE cleanup (see Table 1).
Sample Preparation with Supelclean™ EZ-POP NP Tube (No saponification)
- Weigh ~0.45 g (500 μL, accurate to 0.001 g) of extra-virgin olive oil into a 1.5 mL centrifuge tube with a stopper. For spiked samples, pipette 10 μL of PAH WS to the sample and vortex for 30 seconds.
- Add 40 μL of IS WS, vortex for 30 seconds, and use the resulting sample for SPE cleanup (see Table 2).
The conditions used for GC-MS/MS analysis of all samples are combined in Table 3.
Acceptance Criteria as described in GB 5009.265-2021
- Linear correlation coefficient: R2 > 0.99
- Method analyte recovery: 70 to 130%
- Repeatability: RSD < 20%
Results & Discussion
The chromatographic result for the analysis of a standard mixture of 19 PAH compounds at 40 ng/mL on the ionic liquid SLB®-ILPAH capillary column is shown in Figure 1. GC-MS/MS data for olive oil spiked with twelve PAHs and seven deuterated PAH internal standards, respectively, are displayed in Figures 2 and 3. The SLB®-ILPAH provided satisfying resolution (Figure 1 & 3) for critical sets of PAHs.
Respective chromatography data of the twelve PAH MRM (multiple reaction monitoring) transitions and retention times are listed in Table 5.
![Chromatogram (GC-MS/MS) of a mixture of 19 PAHs (PAH standard solutions - PAH SS) A chromatogram from a GC-MS/MS analysis, displaying the retention times and intensities of a mixture of 19 polycyclic aromatic hydrocarbons (PAHs). The x-axis represents retention time in minutes, ranging from 15 to 35 minutes, while the y-axis represents intensity, labeled as counts multiplied by 100,000. The chromatogram features multiple colored peaks, each corresponding to a specific compound and MRM transition. Key regions of interest are magnified in two insets, highlighting distinct peaks between 22.3 and 22.8 minutes and between 26.5 and 27 minutes. Peaks in the lower main plot are color-coded, including a tall red peak around 19 minutes and a cluster of overlapping peaks in blue, green, yellow, and pink around 30 minutes. The first inset highlights peaks labeled as compounds such as Benz[a]anthracene, Chrysene-d12 (IS), Chrysene, and Benz[a]anthracene-d12 (IS), with curves in blue, yellow, red, and other shades. The second inset shows additional peaks corresponding to Benzo[b]fluoranthene, Benzo[b]fluoranthene-d12 (IS), Benzo[k]fluoranthene, and Benzo[j]fluoranthene, with overlapping lines in pink, green, yellow, and blue. The background is white, and the magnified areas are connected to their corresponding regions in the main chromatogram with black lines for clarity.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/food-and-beverage-testing-and-manufacturing/chemical-analysis/figure-1-chromatogram-19-pah-standard-solutions/figure-1-chromatogram-19-pah-standard-solutions.jpg)
Figure 1.Chromatogram (GC-MS/MS) of a mixture of 19 PAHs (PAH standard solutions - PAH SS). The concentration of twelve undeuterated PAHs was 40 ng/mL, and the concentration for seven deuterated PAH internal standards was 100 ng/mL. (Peak IDs: 2 Benz[a]anthracene, 3 Chrysene-d12 (IS), 4 Chrysene, 5 Benz[a]anthracene-d12 (IS), 8 Benzo[b]fluoranthene, 9 Benzo[b]fluoranthene-d12 (IS), 10 Benzo[k]fluoranthene, 11 Benzo[j]fluoranthene)
![Chromatogram (GC-MS/MS) of an olive oil sample spiked with a standard mixture of 12 PAHs A GC-MS/MS chromatogram of an olive oil sample spiked with a standard mixture of 12 PAHs. The x-axis represents retention time in minutes, spanning from 15 to 35 minutes, and the y-axis displays intensity in counts multiplied by 100,000. The main chromatogram includes several distinct peaks, with a red peak prominently visible around 19 minutes, followed by clusters of overlapping peaks in blue, yellow, light blue, pink, and orange between 22 and 35 minutes. Two inset zoomed sections highlight specific regions of the chromatogram. The first inset, located on the left, focuses on peaks between 22.3 and 22.8 minutes, showcasing four labeled peaks corresponding to Benz[a]anthracene, Chrysene-d12 (IS), Chrysene, and Benz[a]anthracene-d12 (IS). The second inset, on the right, highlights peaks between 26.5 and 27 minutes, labeled as Benzo[b]fluoranthene, Benzo[b]fluoranthene-d12 (IS), Benzo[k]fluoranthene, and Benzo[j]fluoranthene, with curves in green, pink, yellow, and blue. Black lines connect the magnified insets to their respective regions on the main chromatogram for clarity](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/food-and-beverage-testing-and-manufacturing/chemical-analysis/figure-2-chromatogram-spiked-olive-oil-sample/figure-2-chromatogram-spiked-olive-oil-sample.jpg)
Figure 2.Chromatogram (GC-MS/MS) of an olive oil sample spiked with a standard mixture of 12 PAHs at a level of 10 μg/kg and sample prep procedure using Supelclean™ EZ-POP NP (Peak IDs: 2 Benz[a]anthracene, 3 Chrysene-d12 (IS), 4 Chrysene, 5 Benz[a]anthracene-d12 (IS), 8 Benzo[b]fluoranthene, 9 Benzo[b]fluoranthene-d12 (IS), 10 Benzo[k]fluoranthene, 11 Benzo[j]fluoranthene)
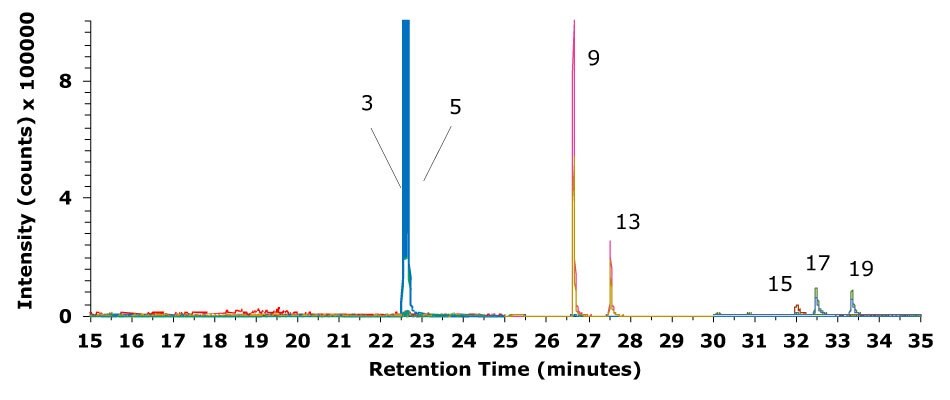
Figure 3.Chromatogram (GC-MS/MS) of an olive oil sample spiked with a mixture of seven deuterated PAH internal standards at a level of 40 μg/kg and sample prep procedure using Supelclean™ EZ-POP NP.
In general, MRM ion pair selection for quantification is done by choosing a mass-to-charge (m/z) difference between the parent and daughter ions greater than 2. However, in this application, some of the analyzed PAH compounds do not meet this prerequisite, because it was not possible to identify ion pairs with a signal response sufficiently high to run quantification experiments. Due to the similarity of their structures, many PAHs also form identical daughter ions. Hence, the choice of these ions can cause errors in quantification. Because of this, the selection of heavier daughter ions (not meeting the criterion mentioned above but yielding higher signal intensities) helped to reduce or avoid interference with other PAHs.
Calibration
The calibration curves for all twelve PAH compounds were obtained by the analysis of six PAH standard solutions (PAH SS with concentrations of 10, 20, 40, 50, 125, and 250 ng/mL for each PAH). The calibration curve for benzo[c]fluorene is displayed in Figure 4 as an example.
![Calibration curve of benzo[c]fluorene The image displays a calibration curve for benzo[c]fluorene with data points and a fitted linear trend line. The x-axis, labeled as "Concentration (µg/mL)," ranges from 0 to 250, while the y-axis, labeled as "Area ratio," spans from 0 to 2.50. The curve features five green circular data points evenly distributed along the fitted line. The linear trend line, also green, extends through the data points and is accompanied by the equation "y = 0.0093x - 0.0833" and a coefficient of determination, "R² = 0.9963," located in the upper right corner. The white background enhances the clarity of the green elements, and the chart has a clean and precise appearance.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/food-and-beverage-testing-and-manufacturing/chemical-analysis/figure-4-calibration-curve-benzo-c-fluorene/figure-4-calibration-curve-benzo-c-fluorene.png)
Figure 4.Calibration curve of benzo[c]fluorene in the concentration range from 10 to 250 ng/mL.
Calibration experiments using the other PAH compounds provided comparable results (see also Table 6). The R2 values, and LOD (limit of detection) and LOQ (limit of quantitation) values in the concentration range from 10 to 250 ng/mL are listed in Table 6. All R2 values are >0.99, which is the requirement of GB 5009.265-2021. The LOD for PAHs was 0.26 - 3.12 μg/kg, and the LOQ was 0.78 – 9.46 μg/kg, respectively.
Repeatability and Recovery
Precision data for the GC-MS/MS method using standard injections with a concentration of PAHs at 40 ng/mL showed RSD values between 1.0 and 4.8 % which is less than 20% as required by GB 5009.265-2021 (Table 7).
The GB 5009.265-2021 suggests styrene divinylbenzene copolymer as the stationary phase to be applied in the SPE process, and Supelclean™ ENVI™-Chrom P SPE tubes meet this requirement. As shown in the sample preparation part, the sample needs to undergo saponification treatment for 1.48 minutes. Because some analytes like benzo[c]fluorene are unstable in the saponification procedure, the reaction time must be controlled. A guideline is giving a 3-minute interval for each gram of sample for saponification. In the experiment, the actual sample size is close to 0.6 g, so the corresponding time was 108 seconds for saponification before running the SPE procedure. This condition is quite demanding, adding complexity and source for errors, and requires therefore a high level of speed and precision from the experimental operator. As a reason of this, we developed a new sample preparation process using Supelclean™ EZ-POP NP SPE tubes to avoid the saponification step. Although both SPE approaches require different working principles, the obtained results are very similar.
The % recovery for the 12 compounds at a spiking level of 10 μg/kg and using the Supelclean™ ENVI™-Chrom P SPE tubes (with prior saponification) and Supelclean™ EZ-POP NP SPE tubes (without saponification) are shown in Table 8. For the Supelclean™ ENVI™-Chrom P SPE tubes they ranged from 90.8 – 122.5%, and RSD were between 1.3 and 8.2%. For the Supelclean™ EZ-POP NP SPE tubes they ranged from 98.4 - 119.3%, and respective RSDs were between 2.5 - 9.2%. The recovery rates and RSD for both SPE approaches met the requirements of GB 5009.265-2021.
A comparison of average % recovery for the PAHs utilizing the two different SPE products is displayed in Figure 5.
![PAH recovery rate in olive oil The bar chart illustrates the recovery rates of PAHs in olive oil using two different methods. The x-axis lists the names of various PAHs, including Benzo[c]fluorene, Benz[a]anthracene, Chrysene, Cyclopenta[cd]pyrene, and others, while the y-axis represents recovery percentages ranging from 0% to 140%. Each PAH category contains two bars: red bars for recovery on Supelclean™ Chrom P after saponification and green bars for recovery on Supelclean™ ENVI™-Chrom P EZ-POP tubes. The bars are closely positioned side by side for comparison, with most values falling between 80% and 120%, indicating high recovery rates. Error bars extend above the tops of the bars, showing the variability in measurements.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/food-and-beverage-testing-and-manufacturing/chemical-analysis/figure-5-pah-recovery-rate/figure-5-pah-recovery-rate.png)
Figure 5.PAH recovery rate (n=3) in olive oil on Supelclean™ Chrom P after saponification red bars) and Supelclean™ ENVI™-Chrom P EZ-POP (green bars) tubes.
Conclusion
The determination of 12 PAHs in olive oil according to the GB 5009.265-2021 method using saponification and clean-up by SPE employing a styrene-divinyl benzene phase, here the suitable Supelclean ENVI-Chrom P, was compared to a new and simpler method using a multilayer SPE tube, the Supelclean™ EZ-POP NP, without prior saponification and with a simpler SPE procedure. After sample preparation, the PAHs were determined by GC-MS/MS using an SLB®-ILPAH column. Both sample preparation approaches showed comparable results and met the acceptance criteria of the GB 5009.265-2021. For most PAHs, the absolute difference in recovery rates between the two sample preparation approaches is less than 8.4%. The only exceptions were chrysene and benzo[k]fluoranthene, for which the difference in recovery rates was 20.1% and 16.4%, respectively. However, since the saponification step is time-consuming and delicate for some analytes not being stable under those conditions, the new method provides a less complex and faster sample preparation approach while still meeting the GB methods acceptance criteria.
More applications on Food & Beverage Testing.
REFERENCES
To continue reading please sign in or create an account.
Don't Have An Account?