Strep•Tag® II Fusion Tag Provides Rapid One-Step Affinity Purification
The Strep•Tag® II/Strep•Tactin® system combines high specificity with gentle elution conditions to provide highly purified, potentially active, recombinant proteins, or protein complexes, after a single purification step. The system includes a variety of vectors, purification products, and detection reagents for rapid affinity purification.
The Strep•Tag® system is based on the reliable biotin/streptavidin binding specificity. The small Strep•Tag® II peptide is an eight amino acid fusion tag with binding specificity comparable to biotin. The small size of the tag reduces potential interference with target protein structure or function. The tag binds to Strep•Tactin® protein, which is an engineered streptavidin with an optimized Strep•Tag® II peptide binding site. The Strep•Tag® II peptide binds to Strep•Tactin® protein nearly 100 times tighter than it binds to streptavidin, but elutes under gentle, physiological conditions. Rapid, one-step affinity purification of Strep•Tag® fusion proteins can result in active proteins at greater than 95% purity. In a comparison of affinity tags, the Strep•Tag peptide was shown to provide excellent purification, with good yields, at a moderate cost (1). The Strep•Tag® system includes Strep•Tag II® vectors (for expression in bacterial, insect, or mammalian cells), a wide range of Strep•Tactin® affinity purification resins, hardware, and buffers, and Strep•Tag® detection reagents.
New Strep•Tag® vectors
The latest pET (E. coli), pIEx™ (insect cells) and pTriEx™ (multi-system E. coli, baculovirus, and mammalian) fusion protein expression vectors incorporate the Strep•Tag® II peptide coding sequence, which is added to generate an N-terminal fusion tag upstream of an enterokinase (Ek) or HRV 3C (3C) cleavage site, as described in Table 1 and illustrated in Figure 1. These vectors share several additional features: a multiple cloning site (MCS) for traditional cloning, resistance to ampicillin, and an optional C-terminal His•Tag® sequence (with 10 histidine residues). With options for “gentle-elution” tags at both the N-terminus (Strep•Tag® II peptide) and C-terminus (His•Tag® peptide), these vectors are ideal for dual purification strategies designed to isolate fulllength, highly purified fusion proteins. Vectors featuring the 3C protease site (2) also encode an optional thrombin protease site to remove the C-terminal His•Tag® peptide fusion.

Figure 1.Maps for the Strep•Tag® II vectors.
Strep•Tag® II Ek MCS

Strep•Tag® II 3C MCS

Ligation-independent cloning (LIC) options
The pET-51b, pIEx™-8, pIEx-10, pTriEx™-5, and pTriEx-7 vectors are new members of the Radiance™ Cloning System (3), which is based on Ek/LIC cloning. Vectors pET-52b, pIEx-9, and pTriEx-6 are available in a new 3C/LIC format as outlined in Figure 2. These 3C/LIC vectors feature an N-terminal Strep•Tag® II fusion tag, which can be cleaved by 3C protease, and an optimal C-terminal His•Tag® fusion tag, which can be cleaved by thrombin. Vectors are available individually as uncut plasmids or linearized in LIC vector kits. LIC vector kits also include a control insert, competent cells, test plasmid, and additional reagents for efficient directional cloning.
Secretion option
pIEx-10 and pTriEx-7 feature the mouse IgM signal sequence for secretion of fusion proteins in both insect (4) and mammalian cells (5). We have verified fusion protein secretion with our constructs in insect and mammalian cells, and export to the periplasm in E. coli cells (data not shown).
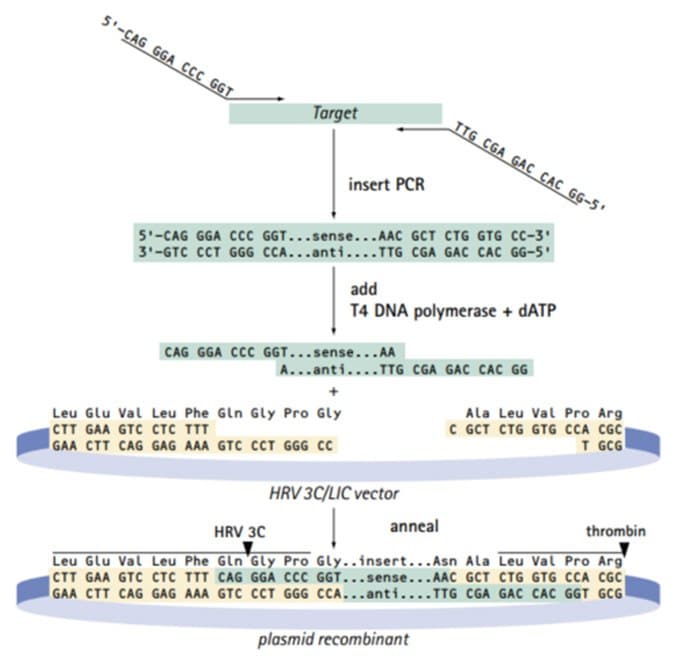
Figure 2.3C/LIC cloning strategy
Strep•Tag® II fusion protein purification
After Strep•Tag® II fusion proteins are expressed, cells are lysed, and the lysate is added to a column or cartridge containing immobilized Strep•Tactin® affinity resin (Figure 3A). The column/cartridge is washed several times with 1X Strep•Tactin® Wash Buffer (150 mM NaCl, 100 mM Tris-HCl, 1 mM EDTA; pH 8.0) to remove nonspecific proteins. Bound Strep•Tag® II proteins are gently eluted with 2.5 mM desthiobiotin. Desthiobiotin is an analog of biotin that competes for the Strep•Tactin® protein binding site (Figure 3C). Because the elution buffer is the wash buffer with desthiobiotin added, and because desthiobiotin is a specific competitor molecule, any proteins nonspecifically bound to the resin generally do not elute with the Strep•Tag® fusion protein.

Figure 3. Schematic of Strep•Tag® II® and One•STrEP™•Tag with Strep•Tactin® affinity purification and column regeneration.
Strep•Tactin® resin regeneration
Strep•Tactin® resin can be regenerated 3 to 5 times. An excess of the yellow azo dye, hydroxyl-azophenylbenzoic acid (HABA), displaces the desthiobiotin from the binding site on the Strep•Tactin® protein (Figure 3D). When HABA binds to the site, it changes color from yellow to red, so regeneration of the column is conveniently indicated by the red color.
Biotin interference
Biotin in cells or culture medium can bind to the Strep•Tactin® protein, reducing the binding capacity of the resin. In many cases the biotin concentration in cell extracts is not high enough to interfere with the purification of the Strep•Tag® fusion protein. However, some cell culture media can contain significant levels of biotin. Avidin, which does not bind to the Strep•Tag® peptide, can be used to bind and “neutralize” any biotin present. To test for possible biotin interference in BacVector® Insect Cell Medium, a transiently expressed Strep•Tag®-Renillaluciferase (RLuc) fusion protein was purified from Sf9 insect cells, either with or without adding avidin to the cells before lysis. Purification results indicated no difference in the amount or the purity of RLuc, with or without added avidin (Figure 4).
Strep•Tactin® resins
Strep•Tactin® resins are available in a variety of prepacked, ready-to-use columns and cartridges or as bulk resin. Strep•Tactin® resins have a binding capacity of 50–100 nmol/mL of resin (up to 3 mg for a 30 kDa protein), depending upon the size of the fusion protein. The dissociation constant (Kd) for Strep•Tag® II is 1 µM. Convenient prepacked Strep•Tactin® Superflow™ Columns (0.2 mL or 1 mL resin) are designed for simple gravity flow purification, and require no additional equipment.
Prepacked Strep•Tactin® Superflow Cartridges (1 mL or 5 mL resin) and Strep•Tactin MacroPrep® Cartridges (1 mL resin) can be used for low pressure liquid chomotography (LPLC) or with syringes. Each resin type exhibits different nonspecific protein binding properties. If nonspecific protein contamination is a problem with a particular host/recombinant protein, using the alternative resin may resolve the problem. The cartridges have a female Luer lock inlet and a male Luer lock outlet. Several different adaptor sets are also available to facilitate connections with commonly used LPLC systems.
For rapid purification of Strep•Tag® proteins from small-scale cultures, Strep•Tactin® SpinPrep™ Kits include spin columns with immobilized Strep•Tactin resin, wash buffer, and elution buffer. SpinPrep™ columns are designed for single use.
To purify samples for high-throughput processing, the Strep•Tactin HT96™ Purification Kit provides Strep•Tactin® resin in a 96-well plate format, with a binding capacity of up to 100 µg Strep•Tag® II protein per well. The kit contains the purification plate, filter plate, wash plate, receiver plate, and wash and elution buffers.
The Strep•Tag II/Strep•Tactin® purification system has been optimized for column purification, so batch methods are not recommended. However, bulk Strep•Tactin® Superflow and MacroPrep Resins offer the flexibility of adjusting the scale of the affinity purification to specific experimental needs. For added convenience, wash, elution, and regeneration buffers are available individually or combined in the Strep•Tactin® Buffer Kit.
Physiological buffers
With the Strep•Tag® II/Strep•Tactin® system, Strep•Tag® II fusion proteins are gently and specifically eluted from the immobilized Strep•Tactin® resin with physiological buffers. Reagents necessary to preserve specific protein structure and activity can be added to the buffers, provided the pH is greater than 7.0.
Denaturing conditions (e.g., 6 M urea, 6 M guanidine hydrochloride) are not compatible with the Strep•Tactin® resin. However, if denaturing conditions are necessary, the two affinity tags (Strep•Tag® II and His•Tag® peptides) on the vectors can be used for dual purification. Initially proteins in denaturing buffer can be purified using His•Bind® purification, which will also concentrate the target protein. After dialysis or buffer exchange, the concentrated protein can be further purified using the Strep•Tactin® resin.
Isolate protein complexes
The gentle purification conditions facilitate the use of Strep•Tag® II fusion proteins to study protein-protein interactions (Figure 3B). The One•STrEP™ Protein Interaction Kit was designed specifically to provide a fast and efficient single-step purification of intact protein complexes. Even weakly and/or transiently associated protein complexes can be isolated. The kit includes the pEXPR-IBA103 Mammalian Expression Vector with a CMV promoter, Strep•Tactin® Superflow™ 0.2 and 1 mL columns, buffers, positive controls, and Strep•Tag® II Antibody HRP Conjugate, for detection.
Detecting Strep•Tag II fusion proteins
Antibodies specifically recognizing the eight amino acids of the Strep•Tag® II fusion tag are available to facilitate detection of Strep•Tag® II fusion proteins. The highly specific Strep•Tag® II Monoclonal Antibody and Strep•Tag® II Antibody HRP Conjugate exhibit no known cross reactivity with other proteins. After reconstituting the lyophilized Strep•Tag® II Monoclonal Antibody, it can be used as a primary antibody at 200 ng/mL to detect 5 ng of Strep•Tag proteins in Western blots. Strep•Tag® II Antibody HRP Conjugate is a single detection reagent that does not require a secondary antibody, and can be used to detect Strep•Tag proteins by ELISA or in Western blots.
To easily verify protein sizes, Strep•Tag II Perfect Protein™ Markers provide a ladder of six Strep•Tag II proteins from 16 to 100 kDa (16, 23.5, 30, 45, 60, 100 kDa). The proteins resolve into sharp, tight bands, which stain evenly when detected with RAPIDStain™ Reagent, Coomassie blue, or with Strep•Tag II antibodies on Western blots.
Introductory kits
Introductory Strep•Tag® II Kits make it easy to start using the Strep•Tag® II system to affinity purify proteins expressed in E. coli. Two kits are available, containing either a pET-51b(+) or a pET-52b(+) vector for cloning the target protein; a Strep•Tactin® Superflow™ column (1 mL), wash, elution, and regeneration buffers for purification; and the Strep•Tag® II Antibody HRP Conjugate for fusion protein detection.
Materials |
|---|
To continue reading please sign in or create an account.
Don't Have An Account?