I6256
2-Iminothiolane hydrochloride
≥98% (TLC), powder
Synonym(s):
2-Thiolanimine hydrochloride, 2IT, Dihydro-2(3H)-thiophenimine hydrochloride, Traut’s reagent
About This Item
Recommended Products
assay
≥98% (TLC)
form
powder
impurities
≤5% Free sulfhydryl groups
mp
198-201 °C (lit.)
solubility
H2O: 100 mg/mL
functional group
thioether
storage temp.
2-8°C
SMILES string
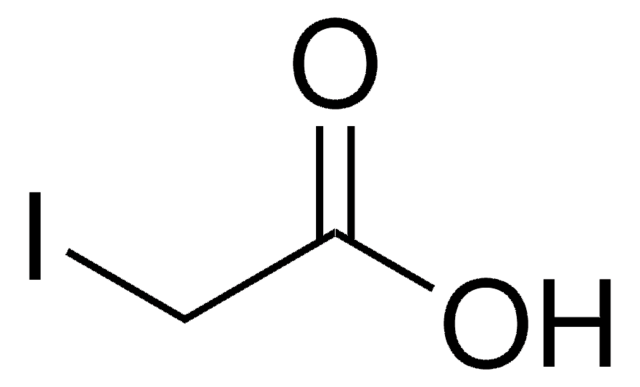
N=C1SCCC1.Cl
InChI
1S/C4H7NS.ClH/c5-4-2-1-3-6-4;/h5H,1-3H2;1H
InChI key
ATGUDZODTABURZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Collagen molecules play a critical role in tissue architecture and strength, and in cell-matrix interactions as insoluble ligands to regulate the diverse phenotypic activities of cells.
Kanjiro Miyata (The University of Tokyo, Japan) provides insights on the rational design of polymeric materials for “smart” oligonucleotide delivery.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service