All Photos(2)
About This Item
Empirical Formula (Hill Notation):
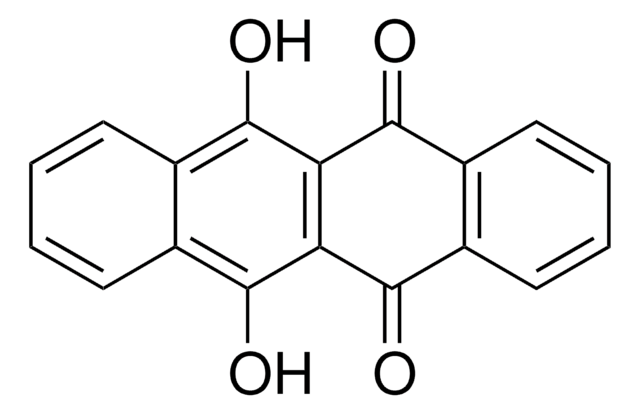
C18H10O2
CAS Number:
Molecular Weight:
258.27
Beilstein/REAXYS Number:
1880180
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
282-286 °C (dec.) (lit.)
functional group
ketone
SMILES string
O=C1c2ccccc2C(=O)c3cc4ccccc4cc13
InChI
1S/C18H10O2/c19-17-13-7-3-4-8-14(13)18(20)16-10-12-6-2-1-5-11(12)9-15(16)17/h1-10H
InChI key
LZPBKINTWROMEA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The electronic structure of the lowest excited triplet state of 5,12-naphthacenequinone was studied using pulsed electron nuclear double resonance and continuous-wave time-resolved EPR (cw-TREPR).
Application
5,12-Naphthacenequinone was used to study the phototransformation of phenol in aqueous solution.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Valter Maurino et al.
Physical chemistry chemical physics : PCCP, 13(23), 11213-11221 (2011-05-17)
The phototransformation of phenol in aqueous solution was studied with different quinoid compounds, which are usually detected on atmospheric particulate matter: 2-ethylanthraquinone (EtAQ), benzanthracene-7,12-dione (BAD), 5,12-naphthacenequinone (NQ), 9,10-anthraquinone (AQ), and 2,6-dihydroxyanthraquinone (DAQ). All the studied quinones were able to sensitise
Takuji Shimokage et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(6), 1201-1208 (2002-05-08)
Continuous-wave time-resolved EPR (cw-TREPR) and pulsed electron nuclear double resonance (ENDOR) studies have been carried out to clarify the electronic structure of the lowest excited triplet (Tl) state of 5,12-naphthacenequinone (5,12-NpQ) as well as 1,4-anthraquinone (1,4-AQ) and 6,13-pentacenequinone (6,13-PeQ). The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service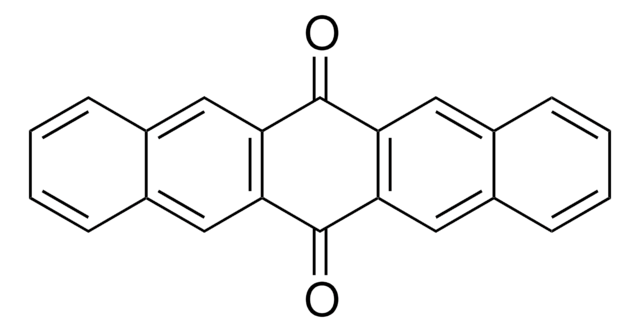




![9,11,20,22-TETRAPHENYLTETRABENZO[A,C,L,N]PENTACENE-10,21-DIONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/202/902/2e5e4f51-7577-47a6-8ced-ecc3534b3a79/640/2e5e4f51-7577-47a6-8ced-ecc3534b3a79.png)


