All Photos(1)
About This Item
Empirical Formula (Hill Notation):
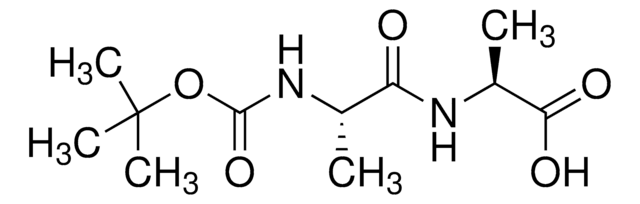
C6H12N2O3
CAS Number:
Molecular Weight:
160.17
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
D-Ala-D-Ala,
assay
≥99%
form
powder
color
white to off-white
storage temp.
−20°C
SMILES string
C[C@@H](N)C(=O)N[C@H](C)C(O)=O
InChI
1S/C6H12N2O3/c1-3(7)5(9)8-4(2)6(10)11/h3-4H,7H2,1-2H3,(H,8,9)(H,10,11)/t3-,4-/m1/s1
InChI key
DEFJQIDDEAULHB-QWWZWVQMSA-N
Related Categories
Application
- Binding Mode-Based Physicochemical Screening Method Using d-Ala-d-Ala Silica Gel and Chemical Modification Approach to Facilitate Discovery of New Macrolactams, Banglactams A and B, from Nonomuraea bangladeshensis K18-0086.: Describes a novel screening method employing D-Ala-D-Ala silica gel to discover new macrolactams with potential antibacterial properties. This technique aids in identifying compounds that inhibit bacterial cell wall synthesis (Kimishima et al., 2024).
Biochem/physiol Actions
D-Ala-D-Ala is found in the stem termini of peptidoglycan side-chain pentapeptide found in the cell walls of gram positive bacteria. The D-ala-d-ala stem termini is the site of interaction of glycopeptide antibiotics such as vancomycin and teicoplanin. D-ala-D-ala is a substrate used to study kinetics of UDPMurNAc-tripeptide D-alanyl-D-alanine-adding (ligase) enzyme.
D-Ala-D-Ala, a terminus moiety of bacterial peptidoglycans, is used for affinity chromatography and binding mechanism studies of antibiotics such as teicoplanin, ristocetin, vancomycin.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Tytgat et al.
Current medicinal chemistry, 16(20), 2566-2580 (2009-07-16)
DD-ligases catalyze the synthesis of the D-Ala-D-Ala and D-Ala-D-Ser dipeptides or the D Ala-D-Lac depsipeptide in an early step of peptidoglycan synthesis. Their function is essential for bacterial growth and specific to bacteria, making them attractive targets for the development
Gareth A Prosser et al.
Antimicrobial agents and chemotherapy, 60(10), 6091-6099 (2016-08-03)
The increasing global prevalence of drug resistance among many leading human pathogens necessitates both the development of antibiotics with novel mechanisms of action and a better understanding of the physiological activities of preexisting clinically effective drugs. Inhibition of peptidoglycan (PG)
Ivona Pavkova et al.
Frontiers in cellular and infection microbiology, 7, 503-503 (2018-01-13)
The DsbA homolog of
Olatunde P Olademehin et al.
ACS omega, 6(1), 775-785 (2021-01-19)
Vancomycin is a glycopeptide antibiotic produced by Amycolaptopsis orientalis used to treat serious infections by Gram-positive pathogens including methicillin-resistant Staphylococcus aureus. Vancomycin inhibits cell wall biosynthesis by targeting lipid II, which is the membrane-bound peptidoglycan precursor. The heptapeptide aglycon structure
Vivek K Mutalik et al.
PLoS biology, 18(10), e3000877-e3000877 (2020-10-14)
Bacteriophages (phages) are critical players in the dynamics and function of microbial communities and drive processes as diverse as global biogeochemical cycles and human health. Phages tend to be predators finely tuned to attack specific hosts, even down to the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service