CC1048
MMP-9, human, proform
Synonym(s):
Progelatinase B, 92 kDa Type IV Collagenase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
eCl@ss:
32160405
NACRES:
NA.41
Recommended Products
biological source
human
assay
>95% (total protein)
form
liquid
specific activity
>1200 mU/mg (specific activity of activated MMP-9 after trypsin activation)
manufacturer/tradename
Chemicon®
concentration
0.2 mg/mL
NCBI accession no.
UniProt accession no.
shipped in
dry ice
Gene Information
human ... MMP9(4318)
General description
When measured with the peptide substrate dinitrophenyl-Pro-Gln-Gly-Ile-Ala-Gly-Gln-D-Arg [Masui et al., 1977] the specific activity of MMP-9 monomer
Application
ACTIVATION:
An aliquot of 10 μL MMP-9 monomer is mixed with 20 μL trypsin solution (see below) and activation buffer in a total volume of 100 μL. The mixture is incubated for 20 min at 37°C. Thereafter trypsin is inhibited by addition of 10 μL aprotinin solution.
Trypsin solution: 0.50 mg TPCK-trypsin/mL activation buffer. The solution is stored in aliquots at -20°C.
Activation Buffer: 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2.
Aprotinin solution: 1 mg aprotinin/mL activation buffer. The solution is stored at -20°C.
An aliquot of 10 μL MMP-9 monomer is mixed with 20 μL trypsin solution (see below) and activation buffer in a total volume of 100 μL. The mixture is incubated for 20 min at 37°C. Thereafter trypsin is inhibited by addition of 10 μL aprotinin solution.
Trypsin solution: 0.50 mg TPCK-trypsin/mL activation buffer. The solution is stored in aliquots at -20°C.
Activation Buffer: 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2.
Aprotinin solution: 1 mg aprotinin/mL activation buffer. The solution is stored at -20°C.
Unit Definition
Specific Activity: where 1 U is the activity that hydrolyzes 1 mmol peptide (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-Dpa-Ala-Arg) within 1 minute under the assay conditions described by Knight ,et al
Physical form
Provided as a liquid in 50 mM Tris-HCl, pH 7.0, 200 mM NaCl, 5 mM CaCl2, ,1 μM ZnCl2, 0.05% Brij-35, 0.05% NaN3.
Storage and Stability
Maintain frozen at -70°C in undiluted aliquots. The enzyme may be stored at -20°C for several without significant loss of activity. Repeated freezing and thawing should be avoided.
Analysis Note
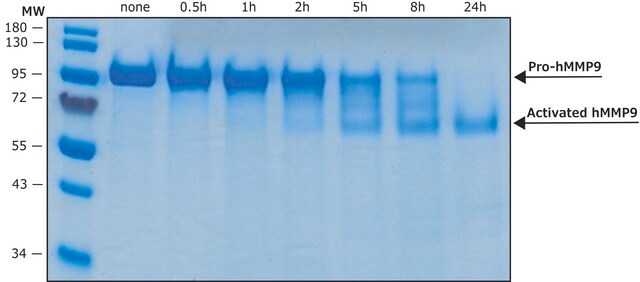
MMP-9 monomer appears as a major band at 92 kDa in non-reducing SDS-PAGE
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status.
Makela, M, et al.
Journal of Dental Research, 73, 1397-1406 (1994)
I E Collier et al.
Genomics, 9(3), 429-434 (1991-03-01)
The 72- and 92-kDa type IV collagenases are members of a group of secreted zinc metalloproteases. Two members of this family, collagenase and stromelysin, have previously been localized to the long arm of chromosome 11. Here we assign both of
Secretion of collagenolytic enzymes by human polymorphonuclear leukocytes.
Hibbs, M S, et al.
Collagen and Related Research, 4, 467-477 (1984)
R W Thompson et al.
The Journal of clinical investigation, 96(1), 318-326 (1995-07-01)
Abdominal aortic aneurysms (AAA) are characterized by disruption and degradation of the elastic media, yet the elastolytic proteinases involved and their cellular sources are undefined. We examined if 92-kD gelatinase, an elastolytic matrix metalloproteinase, participates in the pathobiology of AAA.
Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin.
Goldberg, G I, et al.
The Journal of Biological Chemistry, 267, 4583-4591 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service