137141
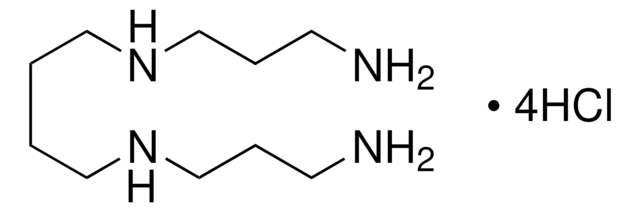
Spermine tetrahydrochloride
EMPROVE® EVOLVE
Pharma Manufacturing
Synonym(s):
N,N′-Bis(3-aminopropyl)-1,4-butanediamine tetrahydrochloride
About This Item
Recommended Products
biological source
synthetic
Quality Level
agency
non-compendial
form
powder
concentration
99.0-101.0 % (w/w) (T)
impurities
≤5000 mg/kg ethanol (HS-GC)
≤890 mg/kg toluene (HS-GC)
mp
310-311 °C (dec.) (lit.)
cation traces
Al: ≤25 mg/kg
Cr: ≤2.5 mg/kg
Cu: ≤25 mg/kg
Fe: ≤130 mg/kg
Ni: ≤2.5 mg/kg
Zn: ≤130 mg/kg
suitability
corresponds for identity (1H-NMR)
application(s)
pharma/biopharma processes
SMILES string
Cl.Cl.Cl.Cl.NCCCNCCCCNCCCN
InChI
1S/C10H26N4.4ClH/c11-5-3-9-13-7-1-2-8-14-10-4-6-12;;;;/h13-14H,1-12H2;4*1H
InChI key
XLDKUDAXZWHPFH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of upstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Trust us to deliver supply chain transparency and reliable sourcing around the globe, streamlining your product qualification with best-in-class regulatory support and service.
Application
Biochem/physiol Actions
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service