All Photos(1)
About This Item
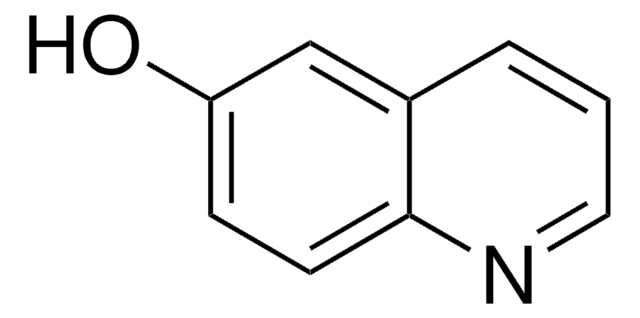
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein/REAXYS Number:
2900
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
200-202 °C (lit.)
SMILES string
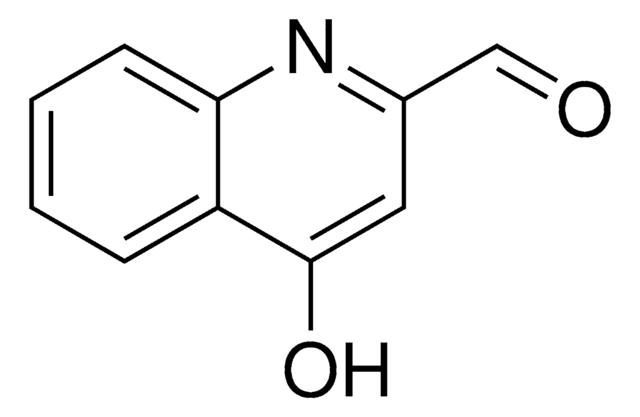
Oc1ccnc2ccccc12
InChI
1S/C9H7NO/c11-9-5-6-10-8-4-2-1-3-7(8)9/h1-6H,(H,10,11)
InChI key
PMZDQRJGMBOQBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Quinolinol (4-quinolone) is a quinolone compound which forms the core moiety of antibacterials such as norfloxacin, nalidixic acid, ciprofloxacin and cinoxacin.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Josip Podobnik et al.
Biomolecules, 10(11) (2020-11-19)
Juvenile delinquency is related to several biological factors, yet very few vulnerability biomarkers have been identified. Previous data suggest that the enzyme monoamine oxidase B (MAO-B) influences several personality traits linked to the propensity to engage in delinquent behavior. Building
Charinya Khamphukdee et al.
Molecules (Basel, Switzerland), 23(9) (2018-09-12)
The previously unreported flavone glycoside, demethyltorosaflavone B (2) and the E-propenoic acid substituted flavone, torosaflavone E (3a), were isolated together with nine previously reported metabolites, including indole-3-carbaldehyde, oleanonic acid, vanillic acid, p-hydroxybenzoic acid, altheranthin (1a), alternanthin B (1b), demethyltorosaflavone D
Óscar M Bautista-Aguilera et al.
International journal of molecular sciences, 21(11) (2020-06-04)
In this communication, we report the synthesis and cholinesterase (ChE)/monoamine oxidase (MAO) inhibition of 19 quinolinones (QN1-19) and 13 dihydroquinolinones (DQN1-13) designed as potential multitarget small molecules (MSM) for Alzheimer's disease therapy. Contrary to our expectations, none of them showed
Takeshi Fuchigami et al.
Nuclear medicine and biology, 35(2), 203-212 (2008-03-04)
High-affinity iodine- and ethyl-C-5 substituted analogs of 4-hydroxy-3-(3-[11C]methoxyphenyl)-2(1H)-quinolone ([11C]4HQ) were synthesized as new positron emission tomography radioligands for the glycine-binding sites of the N-methyl-d-aspartate (NMDA) ion channel. Although both radioligands showed high in vitro specific binding to rat brain slices
Roman Kimmel et al.
Carbohydrate research, 345(6), 768-779 (2010-03-09)
A comparative study for selective glucosylation of N-unsubstituted 4-hydroxyquinolin-2(1H)-ones into 4-(tetra-O-acetyl-beta-D-glucopyranosyloxy)quinolin-2(1H)-ones is reported. Four glycosyl donors including tetra-O-acetyl-alpha-D-glucopyranosyl bromide, beta-D-glucose pentaacetate, glucose tetraacetate and tetra-O-acetyl-alpha-D-glucopyranosyl trichloroacetimidate were tested, along with different promoters and reaction conditions. The best results were obtained
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service