B71608
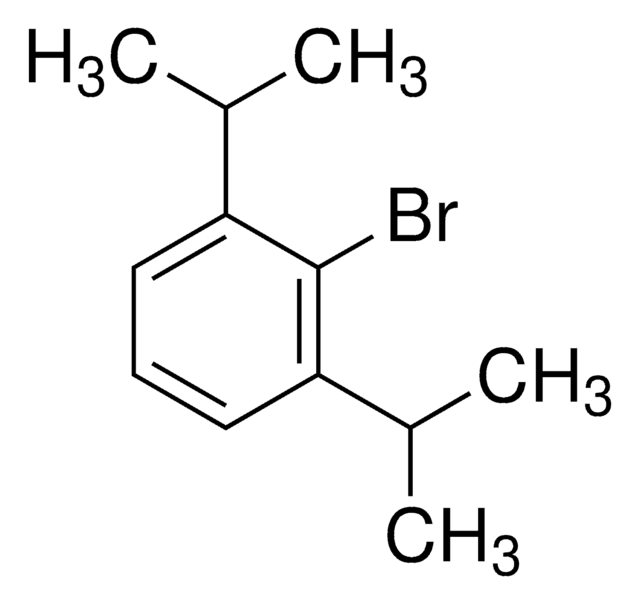
2-Bromomesitylene
98%
Synonym(s):
2-Bromo-1,3,5-trimethylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
1,3,5-(CH3)3C6H2Br
CAS Number:
Molecular Weight:
199.09
Beilstein/REAXYS Number:
1907245
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.552 (lit.)
bp
225 °C (lit.)
mp
2 °C (lit.)
density
1.301 g/mL at 25 °C (lit.)
SMILES string
Cc1cc(C)c(Br)c(C)c1
InChI
1S/C9H11Br/c1-6-4-7(2)9(10)8(3)5-6/h4-5H,1-3H3
InChI key
RRTLQRYOJOSPEA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Bromomesitylene can be used as a reactant for carbon-carbon bond formation and cleavage reactions.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nitrile-group transfer from solvents to aryl halides. novel carbon- carbon bond formation and cleavage mediated by palladium and zinc species
Luo FH, et al.
Organometallics, 17, 1025-1030 (1998)
Karlee L Bamford et al.
Dalton transactions (Cambridge, England : 2003), 49(48), 17571-17577 (2020-11-25)
The synthesis and isolation of the first stable C-B-N-substituted borinium [MesBNiPr2][B(C6F5)4] (2) is described. Compound 2 was shown to react with isothiocyanate and carbodiimides, effecting B-C insertion to afford nitrilium (4) and borenium amidinate salts, respectively. The borinium cation [MesBNiPr2]+
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service