A75900
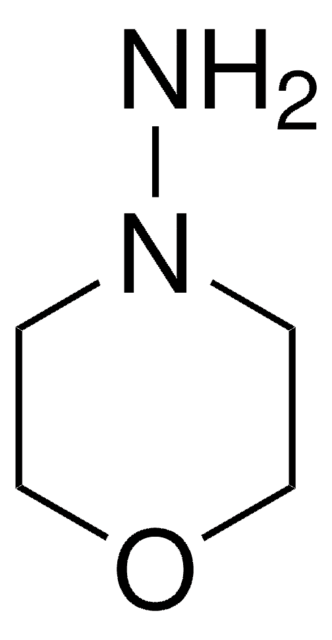
1-Aminopiperidine
97%
Synonym(s):
Pentamethylenehydrazine, Piperidin-1-ylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H12N2
CAS Number:
Molecular Weight:
100.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
liquid
refractive index
n20/D 1.475 (lit.)
bp
146 °C/730 mmHg (lit.)
density
0.928 g/mL at 25 °C (lit.)
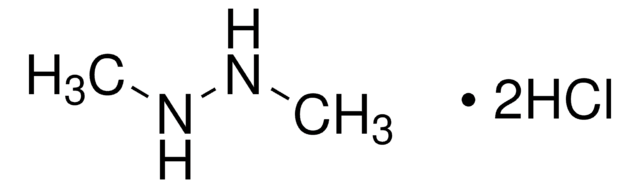
SMILES string
NN1CCCCC1
InChI
1S/C5H12N2/c6-7-4-2-1-3-5-7/h1-6H2
InChI key
LWMPFIOTEAXAGV-UHFFFAOYSA-N
Application
1-Aminopiperidine can be used as a reactant to prepare N-1-piperidinylformamide by reacting with ethyl formate. It is also reacted with aluminum hydride and gallium hydride to form corresponding hydrazides. In the pharmaceutical industry, aminopiperidine is utilized as a building block to synthesize various bioactive molecules.
Reactant for synthesis of:
- CB1 cannabinoid receptor ligands†
- Hydrazones†
- Tetrahydronaphthalene derivatives affecting proliferation and nitric oxide production in LPS activated RAW 264.7 macrophages
- Phosphorus(V) hydrazines
Other Notes
remainder piperidine
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
96.8 °F - closed cup
flash_point_c
36 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aluminum and gallium hydrazides derived from N-aminopyrrole and N-aminopiperidine
Uhl Werner, et al.
Zeitschrift fur Naturforschung B, 61(7), 854-861 (2006)
Fernando C Baltanás et al.
Biochimica et biophysica acta. Reviews on cancer, 1874(2), 188445-188445 (2020-10-10)
SOS1 and SOS2 are the most universal and widely expressed family of guanine exchange factors (GEFs) capable or activating RAS or RAC1 proteins in metazoan cells. SOS proteins contain a sequence of modular domains that are responsible for different intramolecular
Inhibition of metabolism--mediated cytotoxicity by 1,1-disubstituted hydrazines in mouse mastocytoma cells (line P815).
P Wiebkin et al.
Advances in experimental medicine and biology, 136 Pt B, 1067-1075 (1981-01-01)
Synthesis, structure- activity relationship, and evaluation of SR141716 analogues: Development of central cannabinoid receptor ligands with lower lipophilicity
Katoch-Rouse R, et al.
Journal of Medicinal Chemistry, 46(4), 642-645 (2003)
Thomas Lübbers et al.
Bioorganic & medicinal chemistry letters, 17(11), 2966-2970 (2007-04-10)
In a search for novel DPP-IV inhibitors, 2-aminobenzo[a]quinolizines were identified as submicromolar HTS hits. Due to the difficult synthetic access to this compound class, 1,3-disubstituted 4-aminopiperidines were used as model compounds for optimization. The developed synthetic methodology and the SAR
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service