795666
Garg 5-bromo-3,4-pyridyne precursor
95%
Synonym(s):
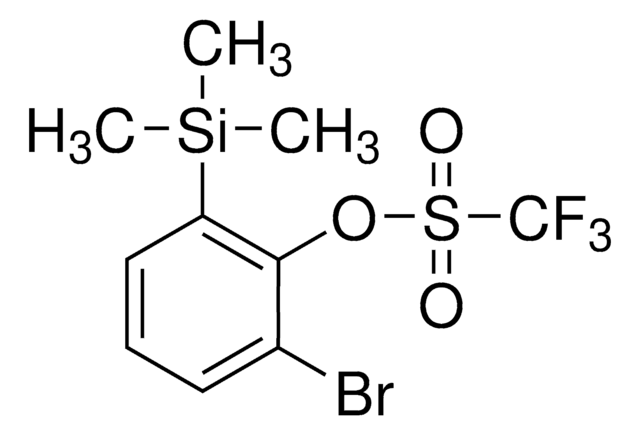
5-Bromo-4-(trimethylsilyl)pyridin-3-yl trifluoromethanesulfonate
About This Item
Recommended Products
Quality Level
assay
95%
form
liquid
density
1.549 g/L at 25 °C
storage temp.
2-8°C
SMILES string
BrC1=CN=CC(S(=O)(C(F)(F)F)=O)=C1[Si](C)(C)C
InChI
1S/C9H11BrF3NO3SSi/c1-19(2,3)8-6(10)4-14-5-7(8)17-18(15,16)9(11,12)13/h4-5H,1-3H3
InChI key
DFTOVRWLXREECB-UHFFFAOYSA-N
Application
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
New and efficient methods for the synthesis of functionalized heterocycles are highly sought after.
Related Content
The Garg group develops methods for the synthesis of natural products and pharmaceuticals. One key method pertains to heterocyclic arynes, such as indolynes and pyridynes, which are generated in situ from silyltriflate precursors under mild fluoride based reaction conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service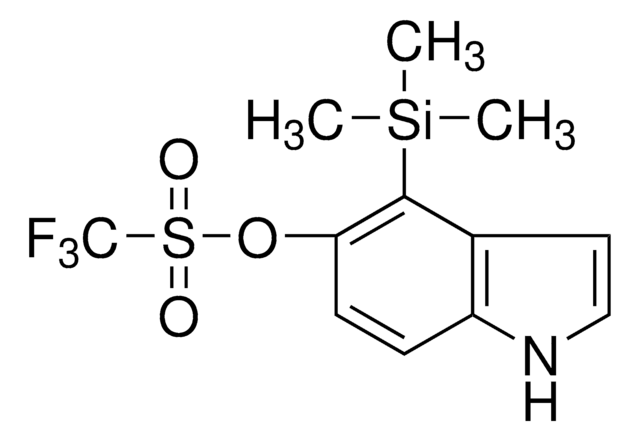

![6-Bromoimidazo[1,5-a]pyridine](/deepweb/assets/sigmaaldrich/product/structures/411/442/0fedbeaf-2927-4ff7-950c-317fda60b64e/640/0fedbeaf-2927-4ff7-950c-317fda60b64e.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

