Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
597988
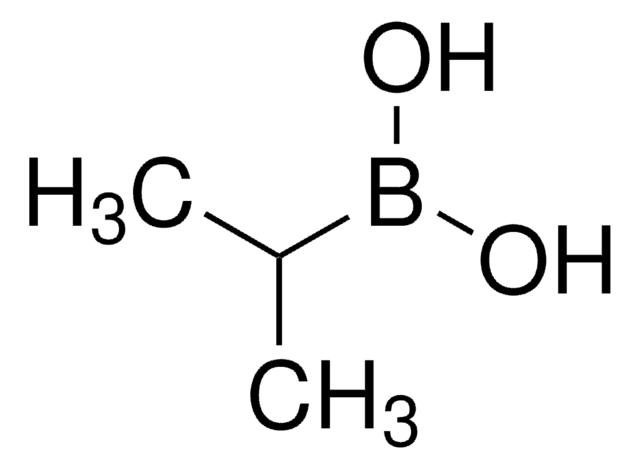
Cyclopropylboronic acid
Synonym(s):
Cyclopropaneboronic acid
Select a Size
Select a Size
About This Item
Recommended Products
form
solid
Quality Level
mp
90-95 °C (lit.)
storage temp.
−20°C
SMILES string
OB(O)C1CC1
InChI
1S/C3H7BO2/c5-4(6)3-1-2-3/h3,5-6H,1-2H2
InChI key
WLVKDFJTYKELLQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Microwave-assisted copper(II)-catalyzed N-cyclopropylation[3]
- Nickel- and copper-catalyzed Suzuki-Miyaura coupling reaction of arenes[4]
- Palladacycle-catalyzed Suzuki-cross coupling of aryl halides with cyclopropylboronic acid[5]
- Palladium(0)-catalyzed cyclopropane C-H bond functionalization[6]
- Palladium-catalyzed decarboxylative coupling[7]
- Palladium-catalyzed ligand-directed oxidative functionalization of cyclopropanes[8]
- Palladium-catalyzed Suzuki coupling reaction[9]
Reagent used in Preparation of
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
What is it about boronic acids that makes them acidic?
1 answer-
Boronic acids have a vacant p orbital. Therefore, despite the presence of two hydroxyl groups, the acidic character of most Boronic acids is that of a Lewis acid.
Helpful?
-
-
What compounds are formed when boronic acids degrade?
1 answer-
Boronic acids are typically very stable. However, if degradation occurs, boronic acid will degrade to corresponding alcohol and boric acid.
Helpful?
-
-
How does the storage temperature relate to shipping conditions?
1 answer-
The storage conditions that a Sigma-Aldrich catalog and label recommend for products are deliberately conservative. For many products, long-term storage at low temperatures will increase the time during which they are expected to remain in specification and therefore are labeled accordingly. Where short-term storage, shipping time frame, or exposure to conditions other than those recommended for long-term storage will not affect product quality, Sigma-Aldrich will ship at ambient temperature. The products sensitive to short-term exposure to conditions other than their recommended long-term storage are shipped on wet or dry ice. Ambient temperature shipping helps to control shipping costs for our customers. At any time, our customers can request wet- or dry-ice shipment, but the special handling is at customer expense if our product history indicates that the product is stable for regular shipment.
Helpful?
-
-
What is the solubility for Product 597988, Cyclopropylboronic acid?
1 answer-
Product No. 597988 should be soluble in methanol at 0.1g in 30 mL (approximately 3 mg/mL).
Helpful?
-
-
What is the pKa of Product 597988, Cyclopropylboronic acid?
1 answer-
The pKa of most boronic acids is approximately 9.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)