All Photos(1)
About This Item
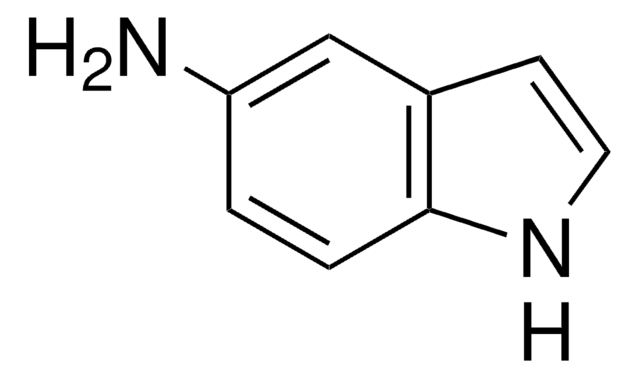
Empirical Formula (Hill Notation):
C8H6IN
CAS Number:
Molecular Weight:
243.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
101-104 °C (lit.)
SMILES string
Ic1ccc2[nH]ccc2c1
InChI
1S/C8H6IN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
InChI key
TVQLYTUWUQMGMP-UHFFFAOYSA-N
General description
5-Iodoindole can be synthesized via nitration of m-toluidine.
Application
5-Iodoindole (5-iodogramine) may be used in the synthesis of the following:
- 3-dimethylaminomethyl-5-iodoindole via reaction with dimethyl amine and formaldehyde
- 5-ethynyl-1H-indole obtained via refluxing with trimethylsilylacetylene in the presence of triethylamine, catalyzed by palladium and copper(I)iodide in acetonitrile
- 5-(3-hydroxyprop-1-enyl)-1H-indole via reaction with allyl alcohol in the presence of triphenyl phosphine, palladium acetate and silver acetate in dimethylformamide
- 5-(3-benzyloxyprop-1-enyl)-1H-indole via reaction with allylbenzyl ether in the presence of triphenyl phosphine, palladium acetate and silver acetate in dimethylformamide
- 5-(2-phenylethynyl)-1H-indole via refluxing with phenylacetylene catalyzed by copper(I)iodide and palladium in the presence of triethylamine in acetonitrile
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Synthesis and biological evaluation of new dipyrrolo [3, 4-a: 3, 4-c] carbazole-1, 3, 4, 6-tetraones, substituted with various saturated and unsaturated side chains via palladium catalyzed cross-coupling reactions"
Henon H, et al.
Bioorganic & Medicinal Chemistry, 14(11), 3825-3834 (2006)
Satish Kumar Rajasekharan et al.
Pesticide biochemistry and physiology, 163, 76-83 (2020-01-25)
Multi-drug resistance in nematodes is a serious problem as lately several resistant phenotypes have emerged following the intermittent usage of synthetic nematicides. Contemporary research continues to focus on developing and/or repurposing small molecule inhibitors that are eco-friendly. Here, we describe
?The synthesis of 5-iodotryptophan and some derivatives"
Harvey GD
Journal of the Chemical Society, 3760-3762 (1958)
Sooyeon Song et al.
Biotechnology and bioengineering, 116(9), 2263-2274 (2019-06-05)
The subpopulation of bacterial cells that survive myriad stress conditions (e.g., nutrient deprivation and antimicrobials) by ceasing metabolism, revive by activating ribosomes. These resuscitated cells can reconstitute infections; hence, it is imperative to discover compounds which eradicate persister cells. By
?Convenient synthesis of 5-iodoindole?
Hydorn.EA
The Journal of Organic Chemistry, 32(12), 4100-4101 (1967)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service