All Photos(1)
About This Item
Linear Formula:
Br(CH3)C6H3NH2
CAS Number:
Molecular Weight:
186.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
95%
refractive index
n20/D 1.6030 (lit.)
bp
105-107 °C/205 mmHg (lit.)
density
1.4780 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Cc1cccc(Br)c1N
InChI
1S/C7H8BrN/c1-5-3-2-4-6(8)7(5)9/h2-4H,9H2,1H3
InChI key
LDUCMSVRKKDATH-UHFFFAOYSA-N
Application
2-Bromo-6-methylaniline can be used as a reactant to synthesize:
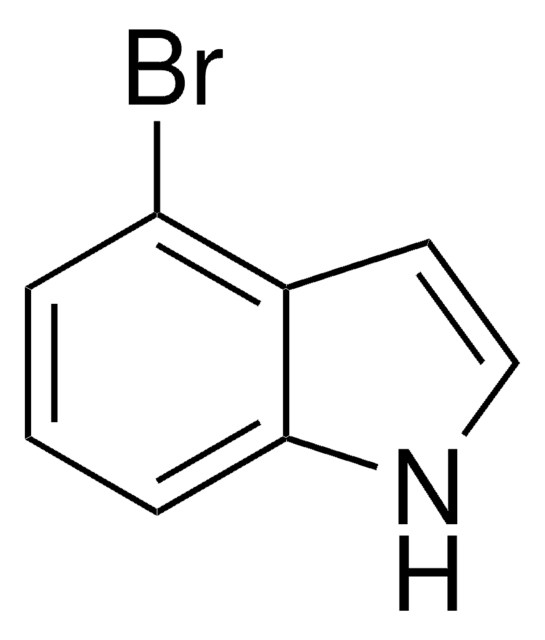
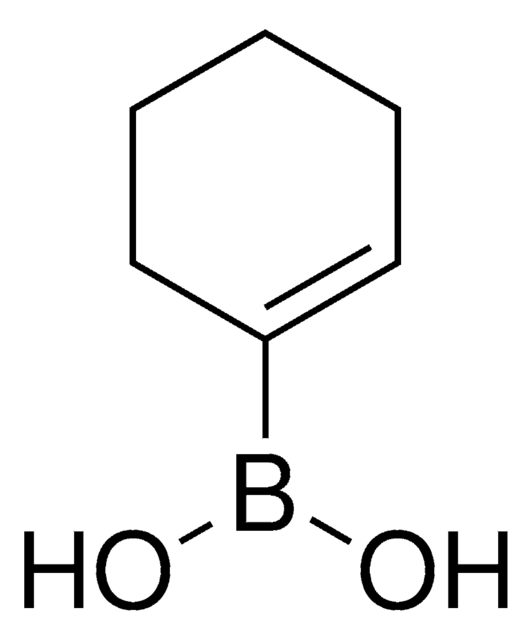
- Alkyl substituted bromoindazole building blocks by Pd-catalyzed Suzuki coupling reaction with various vinyl boronic acids.
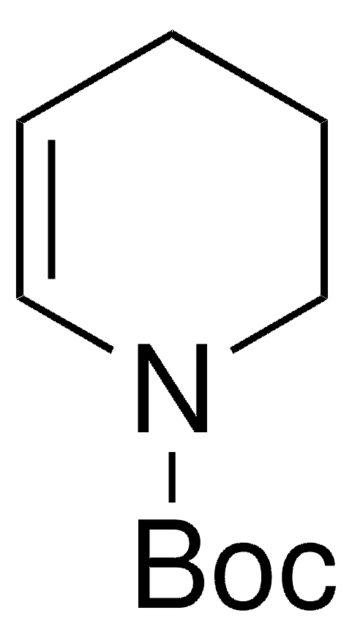
- Difluoropyridoindole via Pd-catalyzed intramolecular Heck reaction with difluoropiperidinone.
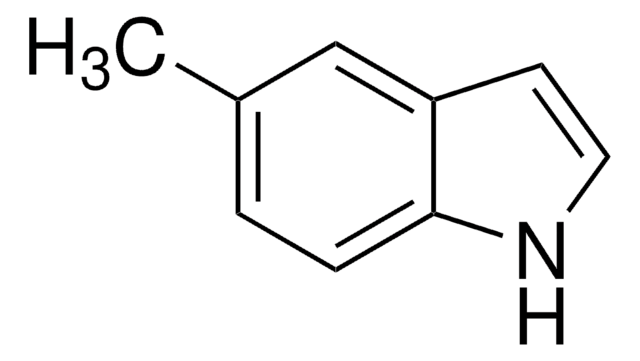
- 4-Methyl-N-phenyl-1H-benzo[d]imidazol-2-amine by one-pot Cu-catalyzed domino C-N cross-coupling reaction with iodobenzene and thiourea.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
215.1 °F - closed cup
flash_point_c
101.7 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and evaluation of indazole based analog sensitive Akt inhibitors
Okuzumi T, et al.
Molecular Biosystems, 6(8), 1389-1402 (2010)
Novel synthesis of 4, 4-difluoropyrido [4, 3-b] indoles via intramolecular Heck reaction
Madaiah M, et al.
Tetrahedron Letters, 54(11), 1424-1427 (2013)
Copper-promoted one-pot approach: Synthesis of benzimidazoles
Boddapati SN, et al.
Molecules (Basel), 25(8), 1788-1788 (2020)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service