559210
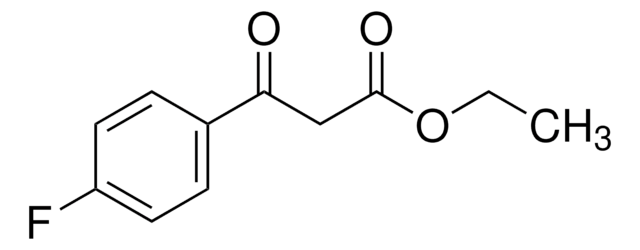
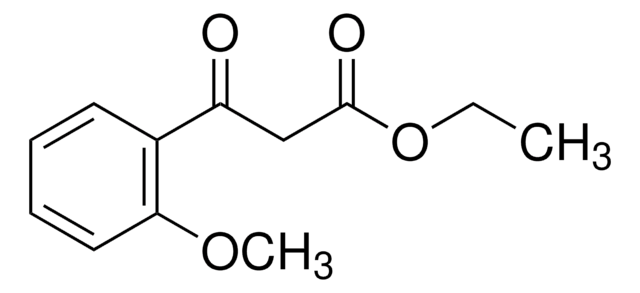
Ethyl (4-chlorobenzoyl)acetate
Synonym(s):
3-(4-Chlorophenyl)-3-oxopropanoic acid ethyl ester, Ethyl 3-(4-chlorophenyl)-3-oxopropanoate, NSC 406743
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4COCH2CO2C2H5
CAS Number:
Molecular Weight:
226.66
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
refractive index
n20/D 1.5500 (lit.)
bp
268-269 °C (lit.)
density
1.218 g/mL at 25 °C (lit.)
functional group
chloro
ester
ketone
SMILES string
CCOC(=O)CC(=O)c1ccc(Cl)cc1
InChI
1S/C11H11ClO3/c1-2-15-11(14)7-10(13)8-3-5-9(12)6-4-8/h3-6H,2,7H2,1H3
InChI key
DGCZHKABHPDNCC-UHFFFAOYSA-N
Application
Ethyl (4-chlorobenzoyl)acetate may be used to synthesize 2-(carboethoxy)-3-(4′-chloro)phenylquinoxaline 1,4-dioxide.
Reactant involved in:
- Tandem condensation-cyclization reactions
- Stereoselective ketonization-olefination of indoles
- Synthesis of antiplasmodial agents
- SIRT 1/2 inhibitor preparation for use as antitumor agents
- Synthesis of mineralocorticoid receptor antagonists
- Intramolecular Michael addition reactions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Comparative Use of Solvent-free KF-Al2O3 and K2CO3 in Acetone in the Synthesis of Quinoxaline 1, 4-Dioxide Derivatives Designed as Antimalarial Drug Candidates.
Lima LM, et al.
Journal of Heterocyclic Chemistry, 42(7), 1381-1385 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service