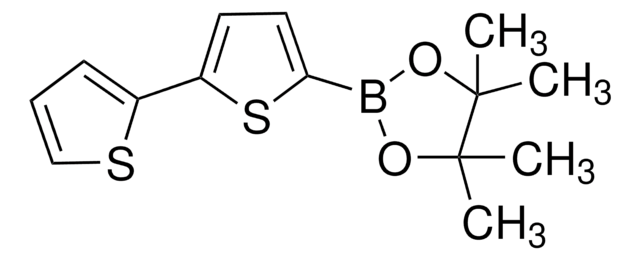
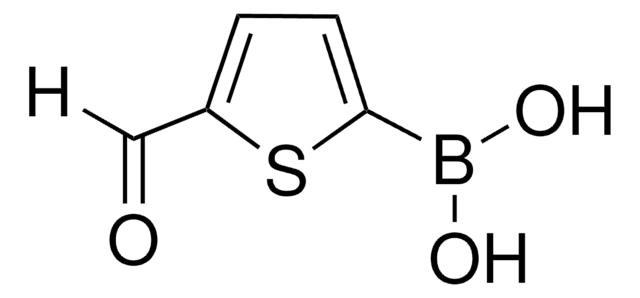
557684
5-Bromo-2-thienylboronic acid
≥95%
Synonym(s):
5-Bromothiophene-2-boronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H4BBrO2S
CAS Number:
Molecular Weight:
206.85
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥95%
form
solid
mp
95-100 °C (lit.)
storage temp.
2-8°C
SMILES string
OB(O)c1ccc(Br)s1
InChI
1S/C4H4BBrO2S/c6-4-2-1-3(9-4)5(7)8/h1-2,7-8H
InChI key
USJPOBDLWVCPGG-UHFFFAOYSA-N
Related Categories
Application
Reactant involved in:
- Suzuki coupling reactions for the synthesis of benzotriazole-containing organic sensitizers and meso-Polyarylamide-BODIPY hybrids
- Suzuki-Miyaura coupling for the synthesis of ratanhine
- Microwave-assisted Sonogashira reactions for the synthesis of ethynylarylboronates
Other Notes
Contains varying amounts of anhydride
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yu Otsuka et al.
Materials (Basel, Switzerland), 13(20) (2020-10-15)
We have established a novel analytical method for solvent polarity on resin surface by combining the synthesis of fluorescent solvatochromic resin with optical waveguide spectrometry. The fluorescent solvatochromic resin was obtained via Suzuki-Miyaura cross-coupling between 4-iodobenzoic acid immobilized on Wang
Articles
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)


![Benzo[b]thien-3-ylboronic acid ≥95.0%](/deepweb/assets/sigmaaldrich/product/structures/136/961/9ddc053e-3519-47d3-be03-95715d131635/640/9ddc053e-3519-47d3-be03-95715d131635.png)