549304
4′-(Methylsulfonyl)acetophenone
97%
Synonym(s):
1-[4-(Methylsulfonyl)phenyl]ethan-1-one, 4-(Methylsulfonyl)acetophenone, NSC 403928
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3SO2C6H4COCH3
CAS Number:
Molecular Weight:
198.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
97%
mp
126-129 °C (lit.)
SMILES string
CC(=O)c1ccc(cc1)S(C)(=O)=O
InChI
1S/C9H10O3S/c1-7(10)8-3-5-9(6-4-8)13(2,11)12/h3-6H,1-2H3
InChI key
KAVZYDHKJNABPC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4′-(Methylsulfonyl)acetophenone may be used in the synthesis of:
- 3-(4-Methylsulfonylphenyl)-4-phenyl-2(5H)-furanone with potent apoptosis-inducing ability.
- Bromo-4-methylsulfonylacetophenone, an intermediate for preparing DL-threo-2-dichloroacetamido-1-(4-methylsulfonylphenyl)-1,3-propanediol.
- 1-N-Substituted-3,5-diphenyl-2-pyrazoline derivatives, which show promising anti-inflammatory activity.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
New Antibacterial Agents. II. An Alternate Synthesis of DL-threo-2-Dichloro-acetamido-1-(4-methylsulfonylphenyl)-1, 3-propanediol1.
Suter CM, et al.
Journal of the American Chemical Society, 75(17), 4330-4333 (1953)
Jiuxiang Zhu et al.
Journal of the National Cancer Institute, 94(23), 1745-1757 (2002-12-05)
The cyclooxygenase-2 (COX-2) inhibitor celecoxib is thought to act as a chemopreventive agent by sensitizing cancer cells to apoptotic signals. Other COX-2 inhibitors, such as rofecoxib, are two orders of magnitude less potent than celecoxib at inducing apoptosis. The molecular
Rossella Fioravanti et al.
European journal of medicinal chemistry, 45(12), 6135-6138 (2010-10-27)
Eighteen new 1-N-substituted-3,5-diphenyl-2-pyrazoline derivatives have been synthesized and cyclooxygenase (COX-1 and COX-2) inhibitory activities have been evaluated. The results of these biological assays showed that all of new derivatives are not endowed with improved anti-inflammatory activity against COX-1, but some
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service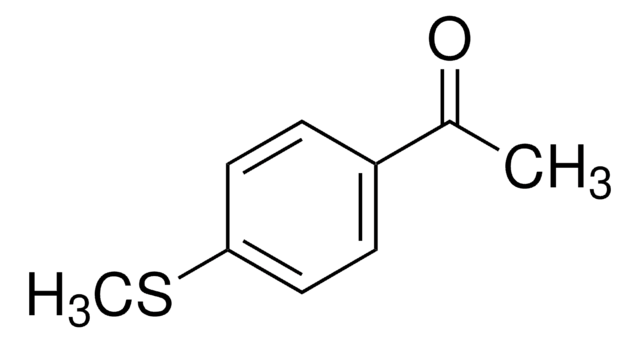
![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)