All Photos(1)
About This Item
Linear Formula:
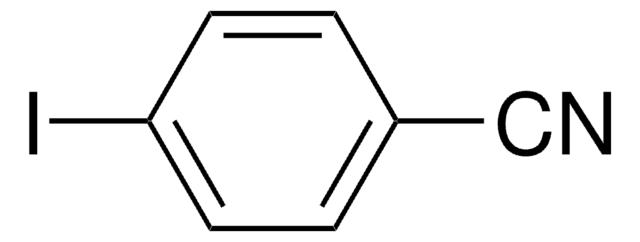
NCC6H4C6H4CN
CAS Number:
Molecular Weight:
204.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
236-240 °C (lit.)
SMILES string
N#Cc1ccc(cc1)-c2ccc(cc2)C#N
InChI
1S/C14H8N2/c15-9-11-1-5-13(6-2-11)14-7-3-12(10-16)4-8-14/h1-8H
InChI key
KAXYYLCSSXFXKR-UHFFFAOYSA-N
General description
4,4′-Biphenyldicarbonitrile (BPCN), also known as 4,4′-dicyanobiphenyl, is a para-disubstituted biphenyl. It can be prepared from benzidine dihydrochloride.3 BPCN belongs to the monoclinic crystal system and P21 space group.
Application
4,4′-Biphenyldicarbonitrile (BPCN, 4,4′-dicyanobiphenyl) may be used in the preparation of:
- [Ag(BPCN)2]PF6 (PF6=Hexafluorophosphate)
- 4,4′-diphenacetylbiphenyl
- triazine-framework-based porous membranes (TFMs)
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation.
Zhu X, et al.
Journal of the American Chemical Society, 134(25), 10478-10484 (2012)
4, 4?-Dicyanobiphenyl.
Britton D and Young Jr VG.
Acta Crystallographica Section E, Structure Reports Online, 59(11), o1849-o1851 (2003)
Crystallization of 4,4?-biphenyldicarbonitrile with silver (I) salts: a change in topology concomitant with a change in counterion leading to a ninefold diamondoid network.
Hirsch KA, et al.
Journal of the Chemical Society. Chemical Communications, 21, 2199-2200 (1995)
Bistetracyclones? and ?Bishexaphenylbenzenes.
Ogliaruso MA, et al.
The Journal of Organic Chemistry, 28(10), 2725-2728 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![(S,S)-(+)-2,6-Bis[2-(hydroxydiphenylmethyl)-1-pyrrolidinyl-methyl]-4-methylphenol 95%](/deepweb/assets/sigmaaldrich/product/structures/126/939/bff1a61c-8335-434f-8da4-9b1929aef17f/640/bff1a61c-8335-434f-8da4-9b1929aef17f.png)